(UroToday.com) The 2024 GU American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium featured an oral abstract prostate cancer session and a presentation by Dr. Christophe Hennequin discussing long-term results of the GETUG-AFU 18 randomized trial of dose escalation (80 vs 70 Gy) combined with long-term androgen deprivation in high-risk prostate cancer. Dr. Hennequin notes that dose escalation in prostate cancer has a long history with extended follow-up:
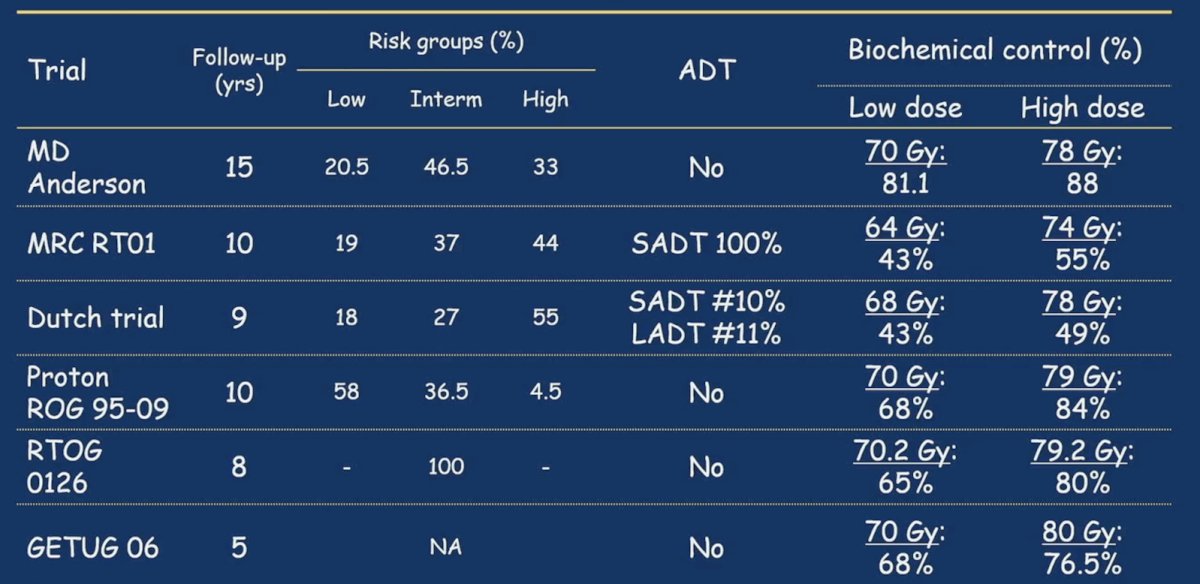
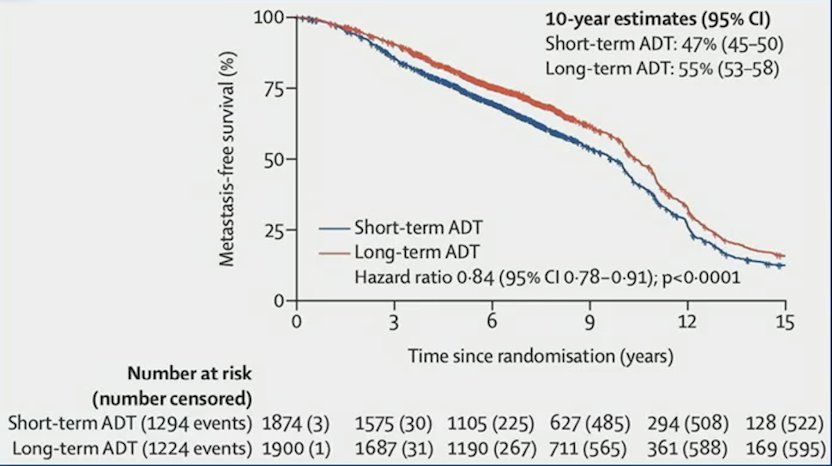
Radiotherapy delivered in combination with ADT improves survival for patients with prostate adenocarcinoma. Recent work from a MARCAP meta-analysis showed that long-term ADT results in better outcomes compared to short term ADT:1
Radiotherapy given at a dose of 80 Gy is generally well tolerated but the occurrence of grade 3-4 toxicities is significantly more frequent than at a dose of 70 Gy. Furthermore, ADT has been reported to increase radiotherapy-related toxicity. Thus, Dr. Hennequin and colleagues aimed to evaluate the efficacy and safety of dose escalation in combination with long term ADT in high-risk prostate cancer patients in the GETUG-AFU 18 randomized trial.
The GETUG-AFU 18 study is a phase III randomized trial. Eligible patients had high-risk (cT3-T4 or PSA≥ 20 ng/ml or Gleason score ≥ 8-10) prostate adenocarcinoma with negative lymph-nodes status on CT-scan or MRI. Patients were either pN0 or cN0 up to 15 mm lymph node diameter on CT or MRI. Patients were randomly assigned 1:1 to dose-escalated radiotherapy (80 Gy) or conventional-dose (70 Gy) with 3 years of ADT in both arms. Randomization was stratified on pelvic lymph node dissection (yes or no) and institution. Pelvic nodal irradiation (46 Gy) was performed for all patients except in case of negative pelvic lymph node dissection. The primary endpoint was biochemical or clinical progression-free survival at 5 years following ASTRO-Phoenix definition. Secondary endpoints were prostate cancer specific survival, overall survival, acute and late toxicity based on CTCAE v3, and quality of life. Exploratory endpoints included clinical relapse-free survival (local, regional, or metastatic) and metastasis free survival. The trial design for GETUG-18 is as follows:
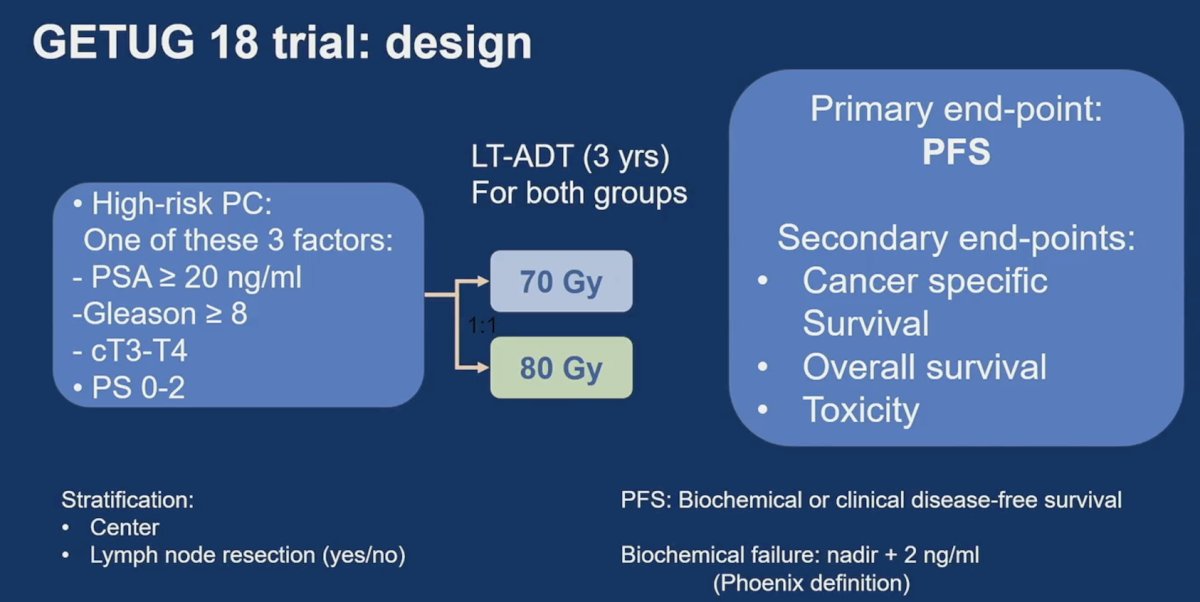
To improve biochemical or clinical progression-free survival from 65% to 75% (HR 0.67), 500 patients were required (a= 5% and 1-b= 80%) with 197 events at 5 years.
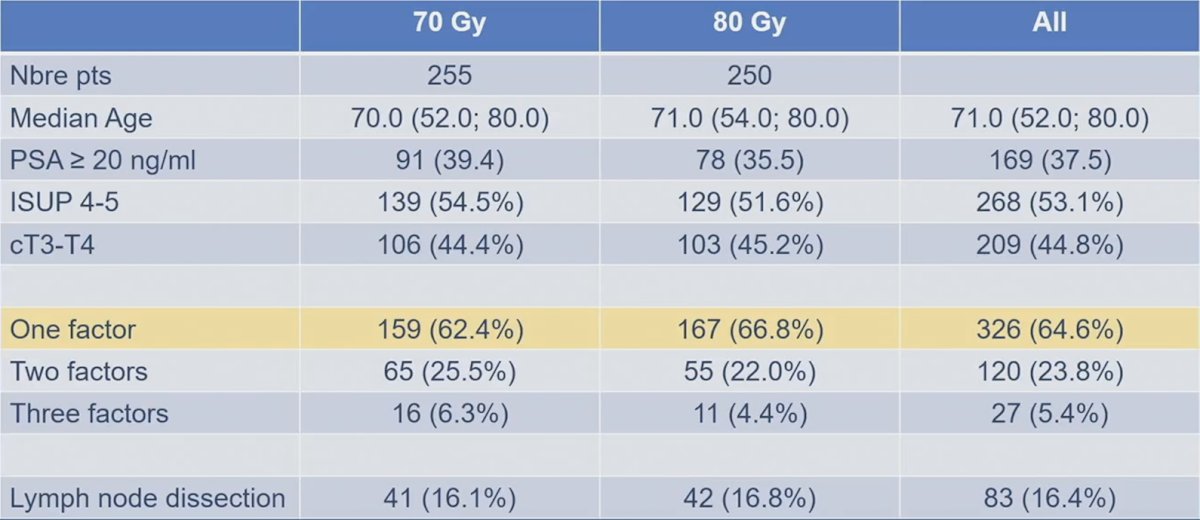
There were 505 patients included in the GETUG-AFU 18 trial between June 4, 2009, through January 24, 2013, at 25 French centers. Key characteristics of the population included a median age of 70.6 years (range 52-80), 53.1% of patients with a Gleason score ≥ 8, median PSA of 13.8 ng/ml (range 0.4-109.9), 62.3% of patients with cT3 disease, and 16.4% of patients having had a pelvic lymph-nodes dissection. There was no imbalance between groups for major prognostic factors:
Over a median follow-up of 9.5 years (95% CI 9.4 – 9.7), the biochemical or clinical progression-free survival was significantly improved in the dose-escalated radiotherapy arm compared with conventional radiotherapy arm (HR 0.56, 95% CI, 0.40-0.76, p = 0.0005):
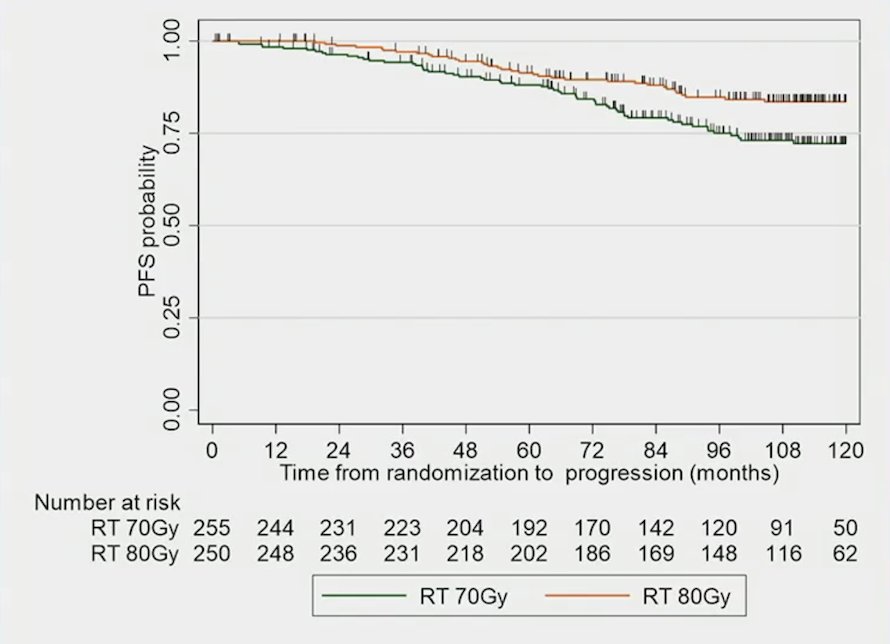
Furthermore, the 5-year biochemical or clinical progression-free survival rate was 91.4% (95% CI, 87.0-94.4%) and 88.1% (95% CI, 83.2-91.6%), and the 10-year biochemical or clinical progression-free survival rate was 83.6% (95% CI, 77.8-88.0%) and 72.2% (95% CI, 72.2-78.0%) in dose-escalated radiotherapy versus conventional radiotherapy, respectively. No prognostic factors were identified for biochemical or clinical progression-free survival.
There were also observed significant differences in prostate cancer-specific survival (HR 0.48, 95% CI, 0.27-0.83, p = 0.0090) favoring dose-escalated therapy:
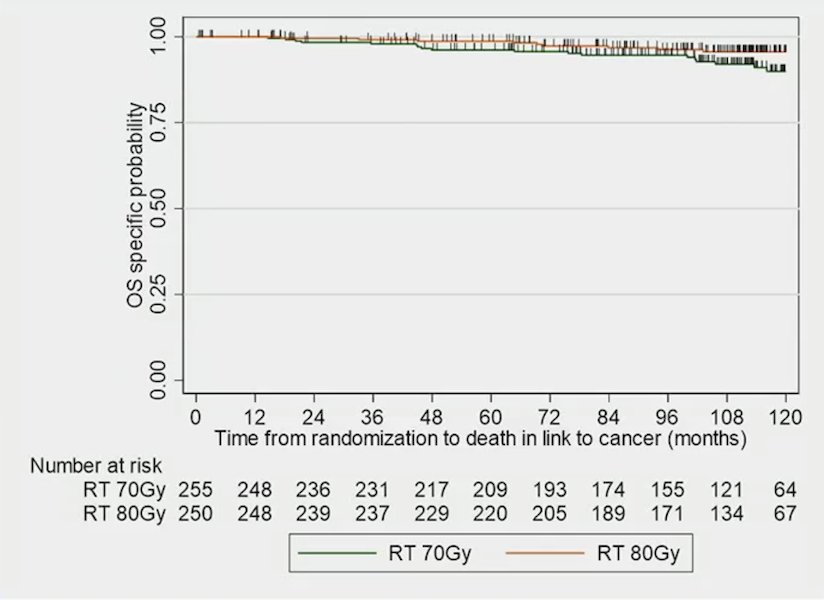
The 5-year prostate cancer specific survival rate was 98.7% (95% CI, 96.2-99.6%) and 96.6% (95% CI, 93.3-98.3%), and the 10-year rate was 95.6% (95% CI, 91.7-97.7%) and 90.0% (95% CI, 84.1-93.8%) in dose-escalated radiotherapy versus conventional radiotherapy, respectively.
Finally, dose-escalated radiotherapy also improved overall survival (HR 0.61, 95% CI, 0.44-0.85, p = 0.0039):
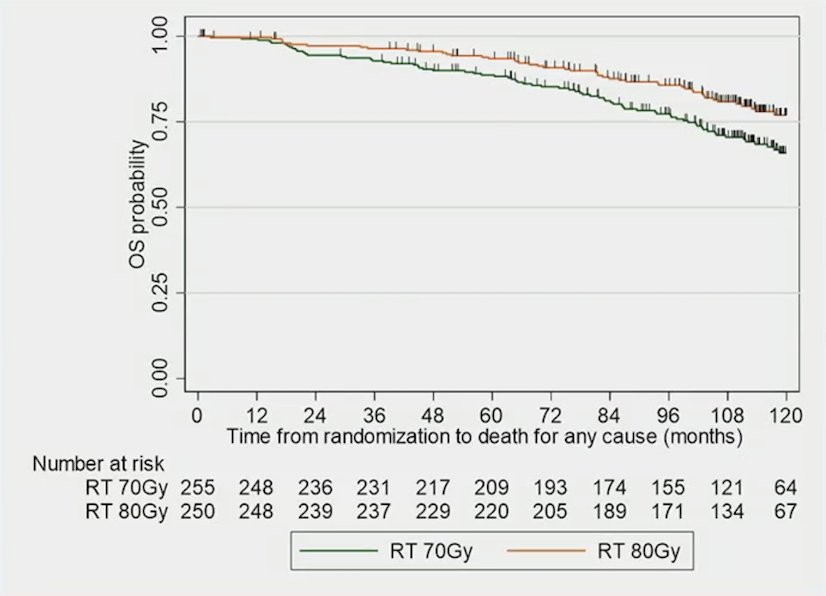
The 5-year overall survival rate was 93.4% (95% CI, 89.5-95.9%) and 88.7% (95% CI, 84.0-92.0%), and the 10-year rate was 77.0% (95% CI, 70.2-82.4%) and 65.9% (95% CI, 58.7-71.1%) in dose-escalated radiotherapy versus conventional radiotherapy, respectively.
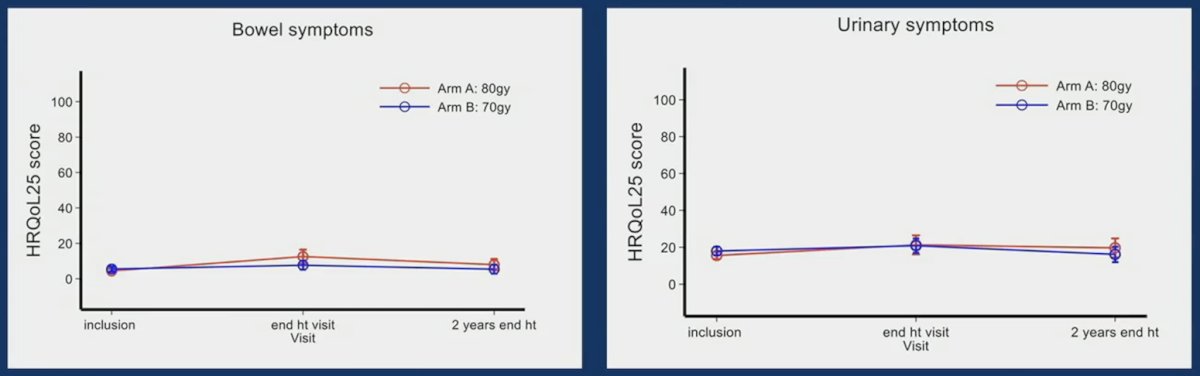
Regarding late toxicities, there was no significant difference between arms with 78.2% and 76.1% of ≥ grade 2 toxicity with dose-escalated radiotherapy and conventional radiotherapy, respectively. For quality of life, there was also no difference between the arms both for bowel and urinary symptoms:
Dr. Hennequin concluded his presentation by discussing long-term results of the GETUG-AFU 18 randomized trial of dose escalation combined with long-term androgen deprivation in high-risk prostate cancer with the following take-home points:
- Even in the case of long-term ADT, higher dose radiotherapy (80 Gy) improves progression free survival, prostate cancer survival and overall survival among patients with high risk prostate cancer
- These efficacy results were achieved with no increase in toxicity
- As such, high dose radiotherapy with long-term ADT is a new standard of care in the management of high risk localized prostate cancer
Presented by: Christophe Hennequin, MD, PhD, Department of Radiation Oncology, Saint-Louis Hospital, Paris, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Kishan AU, Sun Y, Hartman H, et al. Androgen deprivation therapy use and duration with definitive radiotherapy for localized prostate cancer: An individual patient data meta-analysis. Lancet Oncol. 2022 Feb;23(2):304-316.


