(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a prostate cancer poster session. Dr. Alicia Morgan presented the results of a secondary analysis of ARASENS assessing the rate of hospitalization and length of hospital stay during and post-docetaxel for darolutamide-treated patients with metastatic hormone sensitive prostate cancer (mHSPC).
ARASENS is an international, double-blind, phase 3 trial that randomized 1,306 mHSPC patients between November 2016 and June 2018 in a 1:1 fashion to darolutamide 600 mg twice daily or matching placebo in addition to ‘standard of care’ therapy with ADT plus docetaxel. Notably, 85% of patients had de novo metastatic disease. The primary analysis demonstrated that patients in the triplet darolutamide arm had a 32.5% lower rate of death (HR: 0.675, 95% CI: 0.57 – 0.80, p<0.001).1 
In this ARASENS ad hoc analysis, Dr. Morgans and colleagues sought to compare hospitalization rates and length of hospital stay, per patient, due to any reason and due to adverse events, both during and post-docetaxel treatment.
The investigators employed mixed effects negative binomial regression modeling to estimate time-varying rates of hospitalization and length of stay, by patient. Patients with records that began on the same start date were considered duplicates and were condensed into one visit, using the latest end date. Conversely, patients with multiple records with overlapping time periods were combined into one visit.
A constant hospitalization rate assumption was considered inappropriate since docetaxel is administered for six cycles (~4.14 months). As such, hospitalizations that began within and after first 4 months of treatment were grouped together. Conversely, length of hospital stay was assumed to be time invariant.
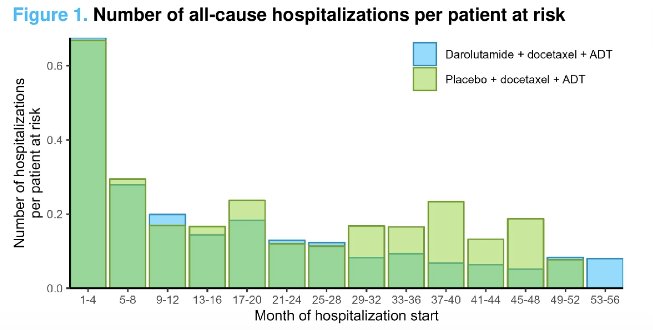
Increased hospitalization rates, due to any reason or secondary to an adverse event, were significantly increased during the 1st four months of treatment (i.e., when patients were receiving docetaxel). During docetaxel treatment, patients receiving darolutamide, compared to placebo, had comparatively lower rates of hospitalization per year due to any reason (0.75 versus 0.91/year) and due to adverse events (0.43 versus 0.50). A similar trend was maintained post-docetaxel treatment (0.31 versus 0.38 and 0.23 versus 0.26, respectively).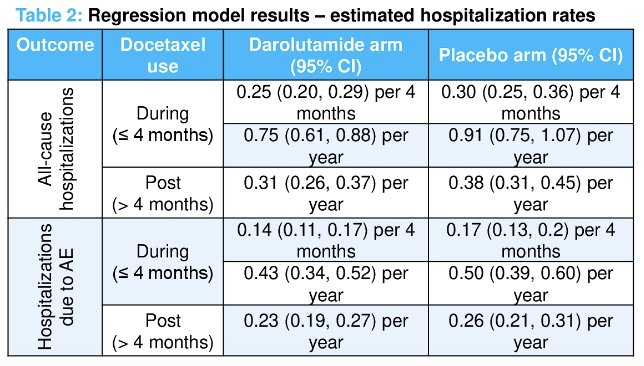
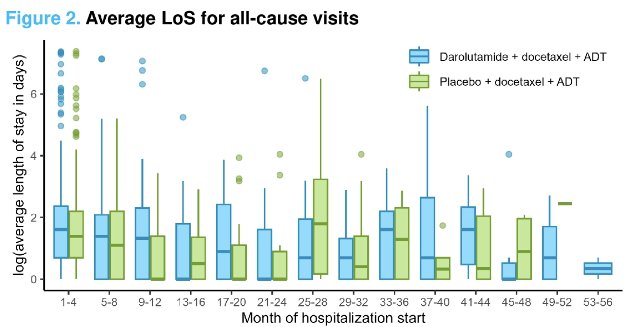
However, darolutamide was associated with a longer length of stay per hospitalization compared with placebo (+1.90 days). Similar results were seen for length of stay due to adverse events (+1.67 days).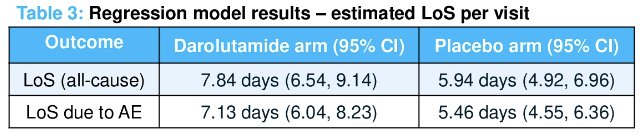
Dr. Morgans concluded that darolutamide addition to docetaxel + ADT for mHSPC patients was associated with lower rates of hospitalization, but marginally longer hospital lengths of stay, compared to placebo. A lower rate of hospitalization was observed post-docetaxel, as opposed to during docetaxel.
Presented by: Alicia K. Morgans, MD, MPH, Associate Professor, Department of Medicine, Medical Director of the Survivorship Program at Dana-Farber Cancer Institute, Massachusetts General Hospital, Boston, MA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:

