(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a prostate cancer poster session. Dr. Esmail Al-Ezzi presented the results of a single center analysis evaluating the survival outcomes of men with metastatic castration-resistant prostate cancer treated with Radium-223, stratified by the presence or absence of homologous recombination repair (HRR) deficiencies.
Radium-223 is a targeted alpha emitter that selectively binds to areas of increased bone turnover in bone metastases and emits high-energy alpha particles of short range (<100 μm). Radium-223 acts as a bone-seeking calcium mimetic and binds into the newly formed bone stroma, especially within the microenvironment of osteoblastic or sclerotic metastases. Double-stranded DNA breaks result secondary to the high-energy alpha-particle radiation. This results in a potent and highly localized cytotoxic effect in the target areas. Furthermore, the short path of the alpha particles also theoretically minimizes the toxic effects on adjacent healthy tissue, including the bone marrow.
In the phase 3 ALSYMPCA trial, the use of Radium-223, compared to placebo, in mCRPC patients with bone-only metastases improved overall survival by 3.6 months (14.9 versus 11.3 months; HR: 0.70, 95%: CI 0.58 to 0.83).1
Given that Radium-223 induces double-strand DNA breaks, Dr. Al-Ezzi and colleagues hypothesized that patients with HRR mutations may exhibit heightened sensitivity to Radium-223, resulting in improved survival outcomes compared to patients without HRR mutations.
This was a retrospective analysis of mCRPC patients with bone-only metastases, with and without HRR mutations, who were treated with Radium-223 at the Princess Margaret Cancer Centre in Toronto, Ontario. Germline and/or somatic DNA sequencing data were identified. Kaplan Meier curves were used to estimate overall and progression-free survivals, with comparisons performed using the log-rank test. Alkaline phosphatase (ALP) and prostate specific antigen (PSA) responses were calculated at 12 weeks post-Radium-223 treatment.
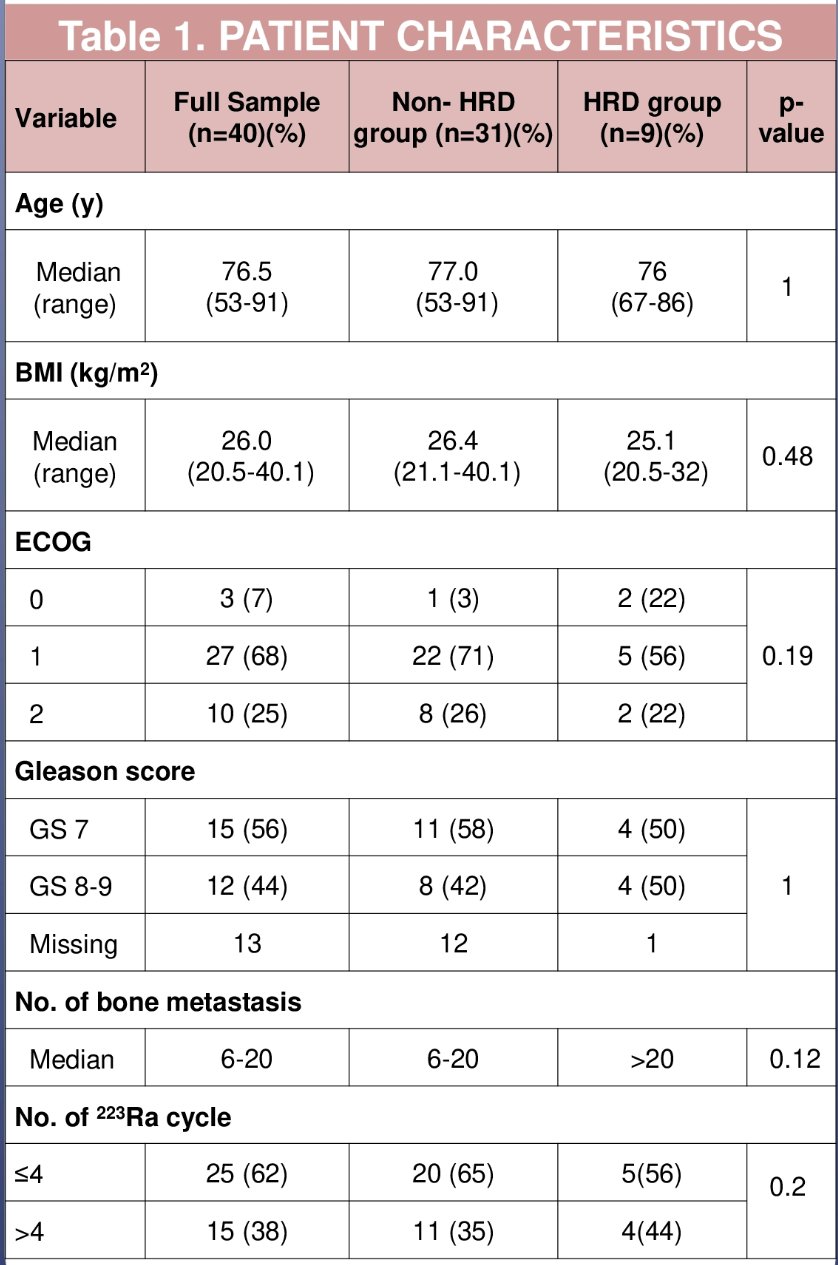
Dr. Al-Ezzi and colleagues identified 40 mCRPC patients who had germline and/or somatic DNA sequencing performed and received Radium-223 between December 2015 and May 2022. The median age at the start of Radium-223 was 76.5 years (range: 66.5 to 80.8 years), and 75% of the cohort had an ECOG performance status of 0 or 1. Overall, 73% of patients receiving Radium-223 were abiraterone- or enzalutamide-pre-treated and 25% had received prior docetaxel.
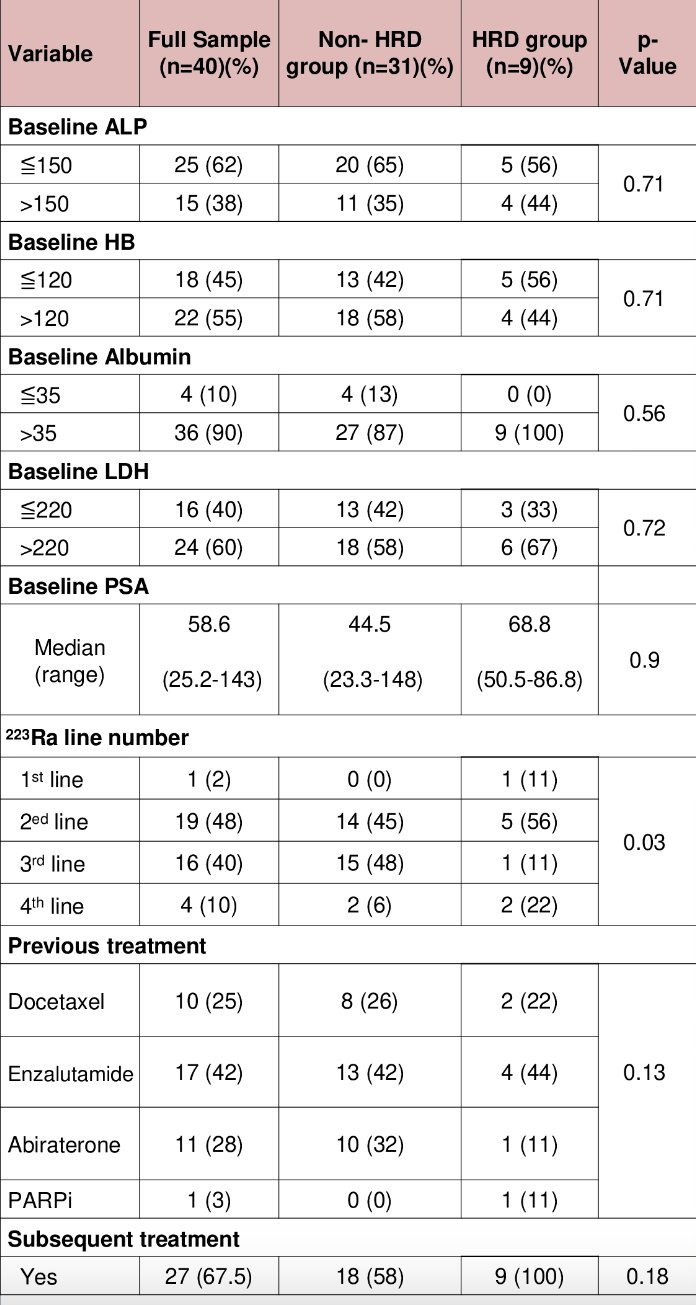
Overall, 22 (54%) received ≥ 4 cycles of Radium-223. The median baseline PSA was 58.6 ng/ml (range: 25.2 to 143 ng/ml) and median baseline ALP was 109 IU/L (range: 71.8 to 200 IU/L). Germline/somatic HRR deficiency mutations were detected in 9/40 (22.5%) patients (BRCA2 [n=6], CHEK2 [n=2], CDK12 [n=1]). Baseline characteristics were well-balanced between HRR mutated and non-mutated groups.
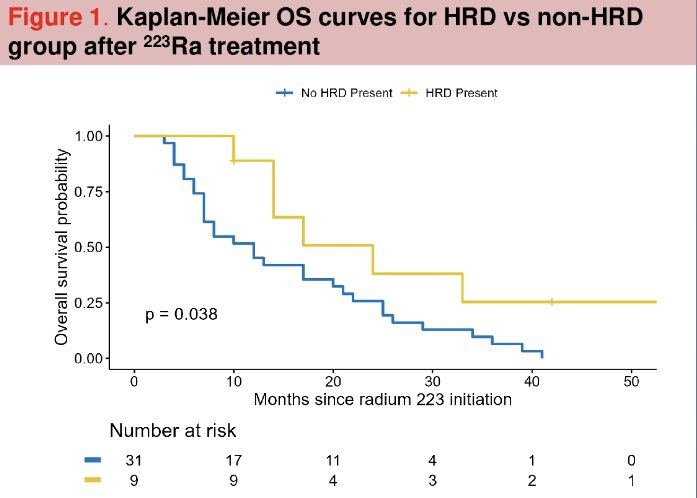
At a median follow-up of 13.7 months, patients in the HRR mutated group had a significantly longer overall survival (median: 24 versus 12 months; p=0.038).
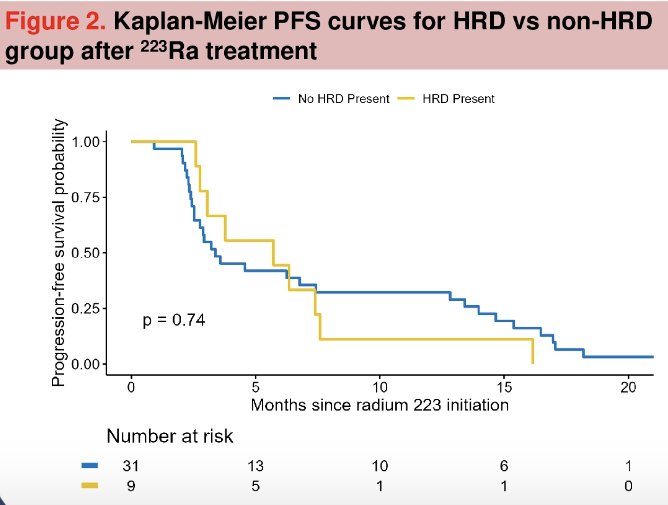
Progression-free survival was similarly superior in the HRR mutated patients, albeit not statistically significant (median: 5.7 versus 3.3 months, p=0.74).
Other outcomes were as follows (HRR mutated versus non-mutated):
- Median time to next treatment: 4.2 versus 3.8 months (p-0.89)
- ALP response: 67% versus 58% (p=0.72)
- PSA response: 33% versus 9.7% (p=0.11)
For all patients with an ALP response, the 3-year survival probability was 33% for HRR mutated patients compared to 11% for those without HRR mutations (p=0.03).
Dr. Al-Ezzi concluded that, despite the small sample size of this study which limited adequate powering of statistical comparisons, this single center analysis suggests that the use of Radium-223 in a mostly abiraterone/enzalutamide and/or docetaxel pre-treated cohort is associated with superior survival outcomes among patients with HRR mutations. Validation of these results in a prospective dataset is required, and whether HRR mutational status has treatment selection implications for other radiopharmaceuticals, such as lutetium-177, remains to be seen.
Presented by: Esmail M. Al-Ezzi, MBBS, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre in Toronto, ON, and Medical Oncology Consultant at King Abdullah Medical City Makkah, Makkah, Saudi ArabiaWritten by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-23.


