(UroToday.com) The 2024 GU ASCO annual meeting featured a renal cell carcinoma session and a presentation by Dr. Martin Voss discussing extended follow-up of the phase 2 KEYNOTE-B61 trial assessing first-line pembrolizumab plus lenvatinib for non–clear cell renal carcinoma. Pembrolizumab + lenvatinib is approved for first-line treatment of advanced/metastatic RCC based on results of the phase 3 KEYNOTE-581/CLEAR study.1 Previously reported results from the phase 2 KEYNOTE-B61 study, with a median follow-up of 15 months, further support the use of pembrolizumab + lenvatinib in the first-line setting specifically across non–clear cell RCC:
- A total of 49.4% of patients (n = 78, 95% CI 41.3-57.4) had a confirmed objective response, with 74.6% of responders remaining in response for >=12 months
- The estimated progression free survival rate was 62.8% and the overall survival rate was 82.2% at 12 months
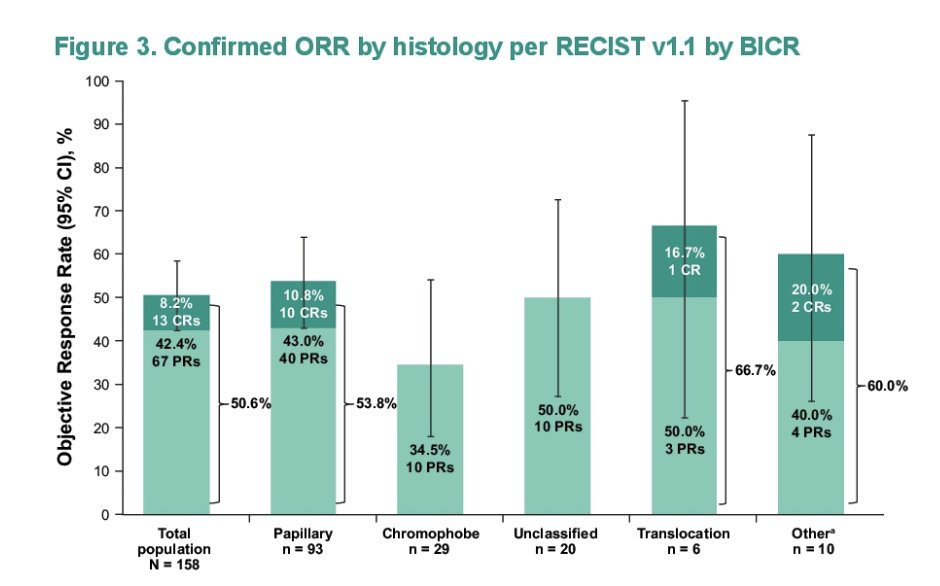
- Efficacy was observed across histologic subtypes with confirmed objective responses in patients with papillary RCC (53.8%), chromophobe RCC (27.6%), unclassified RCC (52.4%), translocation RCC (66.7%), and other histologies
At the GU ASCO 2024 annual meeting, Dr. Voss reported updated results from KEYNOTE-B61 with median follow-up of 23 months. Adults with previously untreated, advanced non–clear cell RCC (histology assessed by investigator) and measurable disease per RECIST v1.1 received pembrolizumab 400 mg IV Q6W for ≤18 cycles (~2 years) + lenvatinib 20 mg orally once daily until intolerable toxicity, progressive disease, or patient withdrawal from the study:
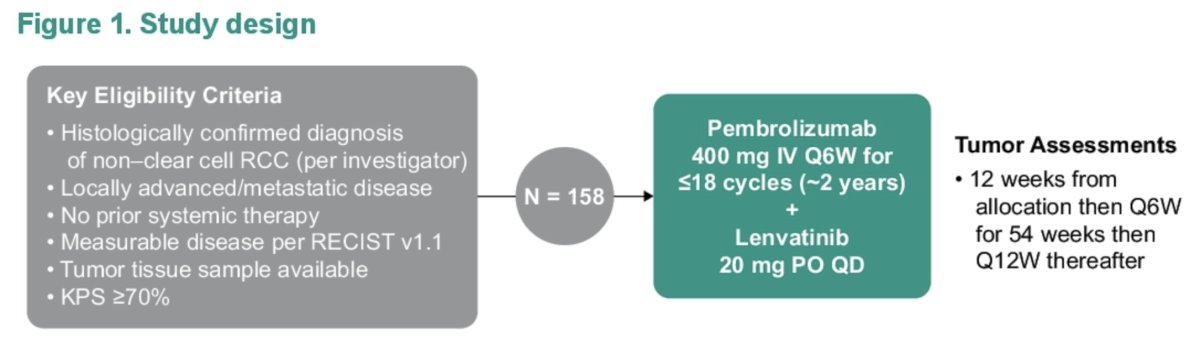
The primary end point was objective response rate per RECIST v1.1 by blinded independent central review. Secondary end points included disease control rate, duration of response, and progression free survival per RECIST v1.1 by blinded independent central review, overall survival, and safety. The median time from first dose to the data cutoff date of July 5, 2023 was 22.8 months (range, 16.6-27.6).
A total of 158 patients received pembrolizumab + lenvatinib. The median age was 60 years (range, 24-87), and the most common histologic variants of RCC were papillary (n = 93; 59%), chromophobe (n = 29; 18%), and unclassified (n = 20; 13%). 86 patients discontinued treatment (most commonly due to progressive disease, n = 56 [35%]). In all patients, objective response rate was 50.6% (95% CI, 42.6-58.7; 13 complete responses [8.2%]; 67 partial responses [42.4%]) and disease control rate was 82.3% (95% CI, 75.4-87.9). The following highlights these outcomes by histology:
The median duration of response was 19.5 months (range, 1.5+ - 23.5+), and an estimated 50.6% of responses had a duration of ≥18 months. In all patients, median progression free survival and overall survival were 17.9 months (95% CI, 15.1-22.1) and NR (95% CI, NR-NR), respectively:
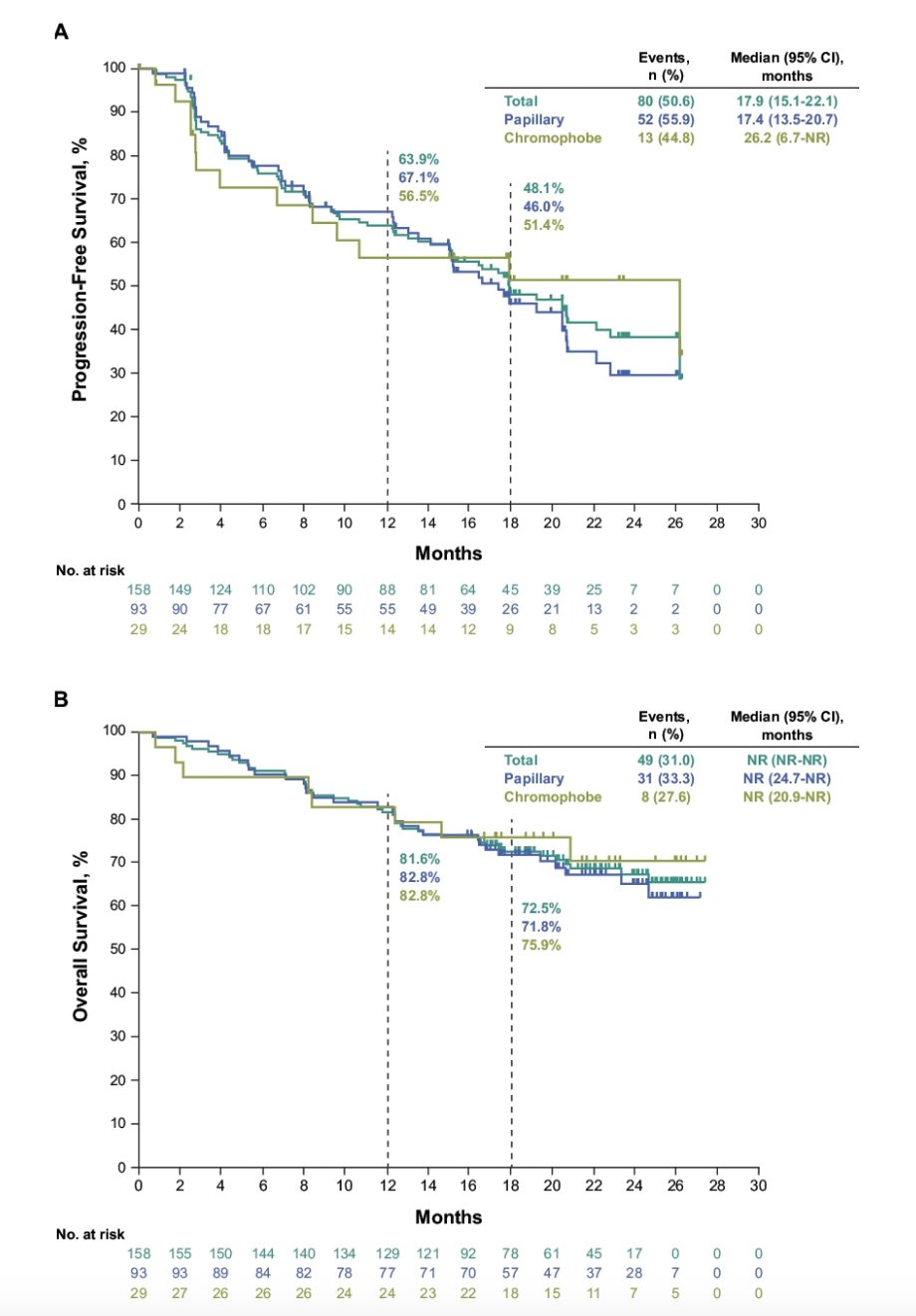
The estimated 18-month progression free survival and overall survival rates were 48% and 73%. Treatment-related adverse events occurred in 151 patients (96%), most commonly hypertension (56%), diarrhea (46%), hypothyroidism (41%), and proteinuria (30%). Grade 3-4 treatment-related adverse events occurred in 92 patients (58%), most commonly hypertension (25%), and diarrhea, proteinuria, and decreased weight (5% each). No deaths due to treatment-related adverse events were reported. Discontinuation due to treatment-related adverse events for pembrolizumab, lenvatinib, or both pembrolizumab and lenvatinib occurred in 15%, 13% and 4% of patients, respectively.
Dr. Voss concluded his presentation discussing extended follow-up of the phase 2 KEYNOTE-B61 trial assessing first-line pembrolizumab plus lenvatinib for non–clear cell renal carcinoma with the following take-home points:
- Consistent with prior reports, pembrolizumab + lenvatinib continued to have durable antitumor activity and a manageable safety profile in patients with advanced non–clear cell RCC
- Pembrolizumab + lenvatinib continued to demonstrate a favorable safety profile
- These results continue to support pembrolizumab + lenvatinib as a first-line treatment option for patients with variant histologies of non–clear cell RCC
Presented by: Martin H. Voss, MD, Memorial Sloan Kettering Cancer Center, New York, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:


