(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a session on optimizing treatment strategies for patients with positive pelvic and/or retroperitoneal lymph nodes. Dr. Robert Svatek discussed whether surgical therapy has a role for the management of urologic oncology patients with node-positive disease.
What do we mean by node positive disease? We must first distinguish between clinical and pathologic nodal positivity. Clinical node positive disease refers to enlarged lymph nodes on cross sectional imaging or PET-avid/enhanced uptake on 18F-FDG-PET. Conversely, pathologic node positive disease refers to that detected on a needle biopsy, frozen section, or permanent section (i.e., lymphadenopathy).
He noted that the node-positive, non-metastatic MIBC cohort is an important one and whose optimal management approach remains an ‘unmet clinical need’. These patients are often excluded from metastatic trials because they do not exhibit distant nodal or visceral metastases. They are also excluded from non-metastatic trials because they are “regional metastases”. Their natural history is also not well-defined, and this is in part due to the current approach to managing such patients being so variable, with different management options including chemoradiotherapy, immunotherapy, surgery, radiation, or any combination of these. As such, there is clearly an opportunity to better study and understand this cohort of patients.
What is the rationale for treating the primary disease site in such patients? There is evidence from multiple solid organ malignancies that the primary site acts as a primer for metastatic disease spread. Thus, by removing the primary site (bladder in this case), Dr. Svatek noted that this may lead to downstream survival benefits – although this is yet to be corroborated by any meaningful evidence in the bladder cancer space.
Conversely, there is a phenomenon of surgery-induced immune dysfunction, that may stimulate metastatic deposits, ultimately leading to adverse effects on survival outcomes. This has been most notably demonstrated in the lung cancer disease space. Dr. Svtak and colleagues evaluated this hypothesis in an implantable bladder cancer mouse model, whereby bladder cancer cells were injected into the rodents’ tails. Mice were then subsequently subjected to a 2 cm laparotomy in the experimental arm versus only general anesthesia in the control arm. As is illustrated below, mice in the surgical group developed a significantly larger volume of lung nodules and had worse cancer-specific survival. Dr. Svatek and colleagues noted that markers of cellular inflammation were also significantly worsened in the surgical mice group.1 Furthermore, surgery impacted the response to anti-PD-L1 therapy, with mice undergoing surgery having decreased responses to anti-PD-L1 therapy and diminished anti-PD-L1 T-cells.
Do these findings have any relevance/significance to humans? In an immune profiling analysis of patients undergoing surgery, Dr. Svatek and colleagues noted that patients post-operatively had a higher proportion of ‘exhausted’ CD4+ T-cells, and there was a non-significant increase in CD8+ T-cells, with decreased proliferative activity for the PD-1+ cells.\
What does the clinical data tell us regarding the optimal management for these patients? A 2016 analysis of the National Cancer Database by Galsky et al. in 2016 demonstrated that node-positive patients who underwent surgery +/- neoadjuvant/adjuvant therapy, had superior overall survival outcomes, compared to chemotherapy only.2 While this analysis was conducted using multivariable modeling that aims to control for the effects of potential known confounders, including tumor, patient, and facility level characteristics, residual confounding secondary to other variables such as frailty, comorbidity, and performance status, known confounders of treatment decision-making, could not be accounted for, which introduces selection bias (systematic error) into such analyses.
A follow-up analysis published in 2021 that employed propensity score matching, compared two groups of node-positive patients, based on treatment received:
- High-intensity: Radical cystectomy + PLND or ‘adequate’ radiotherapy (≥50 Gy) + TURBT
- Conservative group: No local therapy, <50 Gy radiation, or TURBT alone
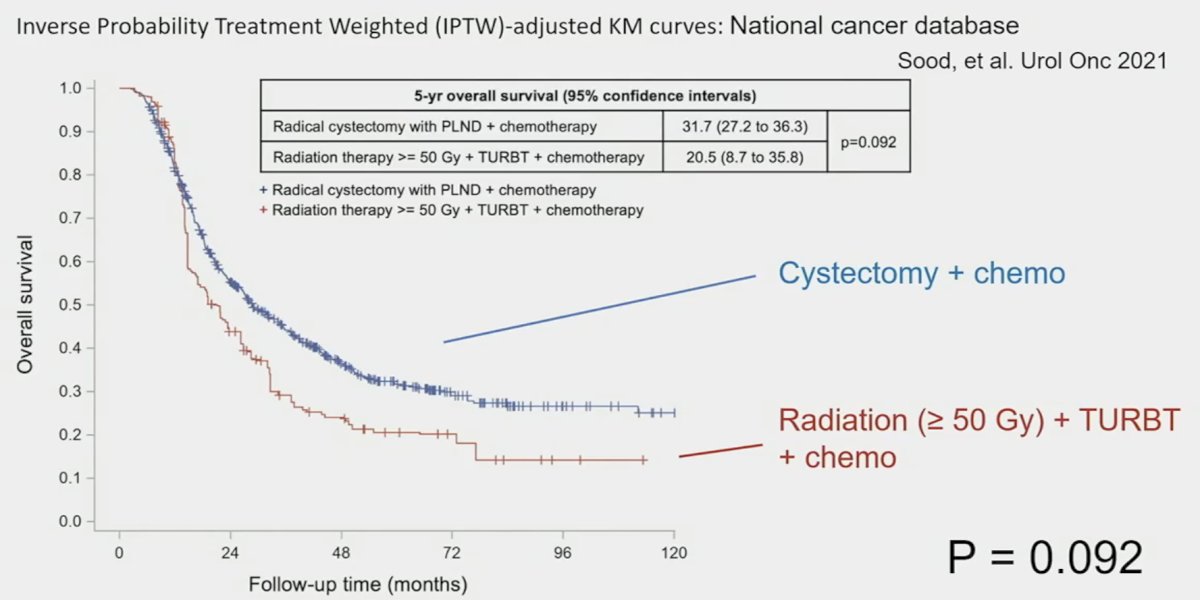
They demonstrated that patients receiving high-intensity therapy had superior 5-year overall survival outcomes (28.4% versus 18.3%, p<0.0001). Furthermore, among patients in the high-intensity group, those receiving cystectomy + chemotherapy fared non-significantly better than those receiving ‘adequate’ radiotherapy + TURBT.3
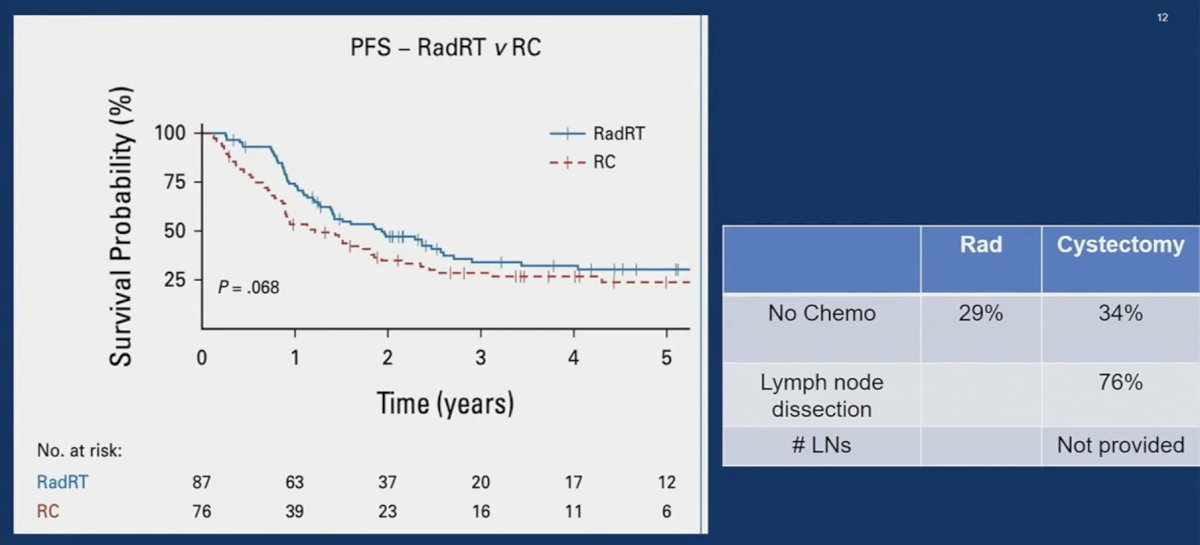
A more recent UK analysis published in 2023 compared radical therapy (cystectomy +/- chemotherapy or radical radiation +/- chemotherapy) to palliative therapy and demonstrated that patients in the radical therapy group had superior progression-free survival outcomes. However, there was considerable selection bias, whereby 51% of patients in the radical therapy group had an excellent performance status of 0, compared to 15% of those in the palliative treatment group. Comparisons of radiotherapy to surgery in this cohort demonstrated non-significant differences slightly favoring radiotherapy (p=0.068); however, Dr. Svatek noted that 34% of patients in the cystectomy arm did not receive chemotherapy, which is currently standard of care, and 24% of radical cystectomy patients did not undergo a lymph node dissection.4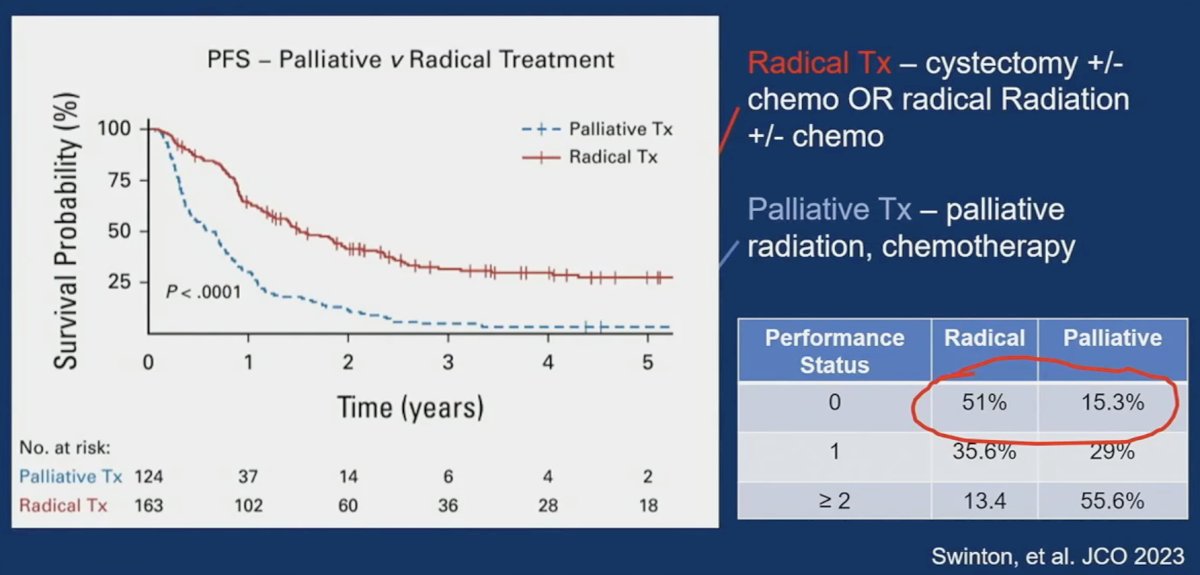
While bladder preservation strategies offer obvious advantages, there are benefits to a palliative cystectomy, particularly with regards to symptom control:
- Gross hematuria, clot retention, blood-loss anemia
- Urinary obstruction - reduce the need for tubes
- Urinary tract infections - especially in the setting of chronic tubes/catheters
- Voiding dysfunction- urinary frequency, urgency, nocturia
- Bladder/pelvic pain
In conclusion, is there a role for surgery in N+ positive patients?
- Pros:
- Disease control in ~30% of patients
- Symptom mitigation, enhanced QoL potentially
- Disease assessment (pathologic confirmation)
- Could potentially mitigate primary tumor influence on metastatic sites
- Cons:
- Surgery-induced immune dysfunction
- Surgery complications affects the ability to deliver other treatment
Presented by: Robert S. Svatek, MD, Professor and Chair, Gary and Glenda Woods President’s Distinguished University Chair in GU Oncology, Department of Urology, UT Health San Antonio, TX
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:- Svatek RS, Ji N, de Leon E, et al. Rapamycin Prevents Surgery-Induced Immune Dysfunction in Patients with Bladder Cancer. Cancer Immunol Res. 2019;7(3):466-475.
- Galsky MD, Strensland K, Sfakianos JP, et al. Comparative Effectiveness of Treatment Strategies for Bladder Cancer With Clinical Evidence of Regional Lymph Node Involvement. J Clin Oncol. 2016;34(22):2627-2635.
- Sood A, Keeley J, Palma-Zamora, et al. High-intensity local treatment of clinical node-positive urothelial carcinoma of the bladder alongside systemic chemotherapy improves overall survival. Urol Oncol. 2022;40(2):62.e1-62.e11.
- Swinton M, Mariam NBG, Tan JL, et al. Bladder-Sparing Treatment With Radical Dose Radiotherapy Is an Effective Alternative to Radical Cystectomy in Patients With Clinically Node-Positive Nonmetastatic Bladder Cancer. J Clin Oncol. 202341(27):4406-4415.


