(UroToday.com) The 2024 GU ASCO annual meeting included a urothelial carcinoma session featuring trials in progress and a presentation by Dr. Syed Hussain discussing the trial design of INVEST, a phase Ib window of opportunity study of atezolizumab administered either intravesically or direct tumor injection in patients with bladder cancer prior to radical cystectomy. High-risk non-muscle invasive bladder cancer is a common and difficult-to-manage disease. The treatment of choice is radical cystectomy, as BCG-unresponsive tumors have a poor prognosis. Radical cystectomy negatively impacts health-related quality of life and therefore clinicians have explored alternative treatments.
The FDA approved systemic pembrolizumab for BCG-refractory carcinoma in situ,1 but uptake is low, reflecting concerns around efficacy and safety for a non-invasive cancer. Dr. Hussain and colleagues hypothesize that intravesical administration of a PDL1 inhibitor could be effective with less systemic toxicity. However, it is unknown whether antibodies delivered via this route can reach the tumour vasculature. INVEST is a phase Ib window of opportunity study aiming to investigate the safety and preliminary activity of both passive instillation and direct injection into the tumor/bladder wall of intravesical atezolizumab prior to radical cystectomy.
Eligible participants (ECOG performance status 0-2) are awaiting radical cystectomy for urothelial cell carcinoma of the bladder (any stage). Those with muscle invasive cancer must be ineligible for or refuse cisplatin based neoadjuvant chemotherapy. Each dose confirmation stage uses a conventional 3+3 design to establish safety based upon dose limiting toxicities. The recommended single dose will be determined first of either 600 mg or 1200 mg (n = 3-12), and this dose will then be evaluated further to determine the recommended multiple dose of either 600 mg or 1200 mg administered weekly for 3-6 weeks (n = 3-12). A dose expansion stage will then treat 10 patients at the recommended multiple dose to further evaluate safety and toxicity. This schema will be followed independently for both the passive instillation and direct injection routes of intravesical atezolizumab administration:
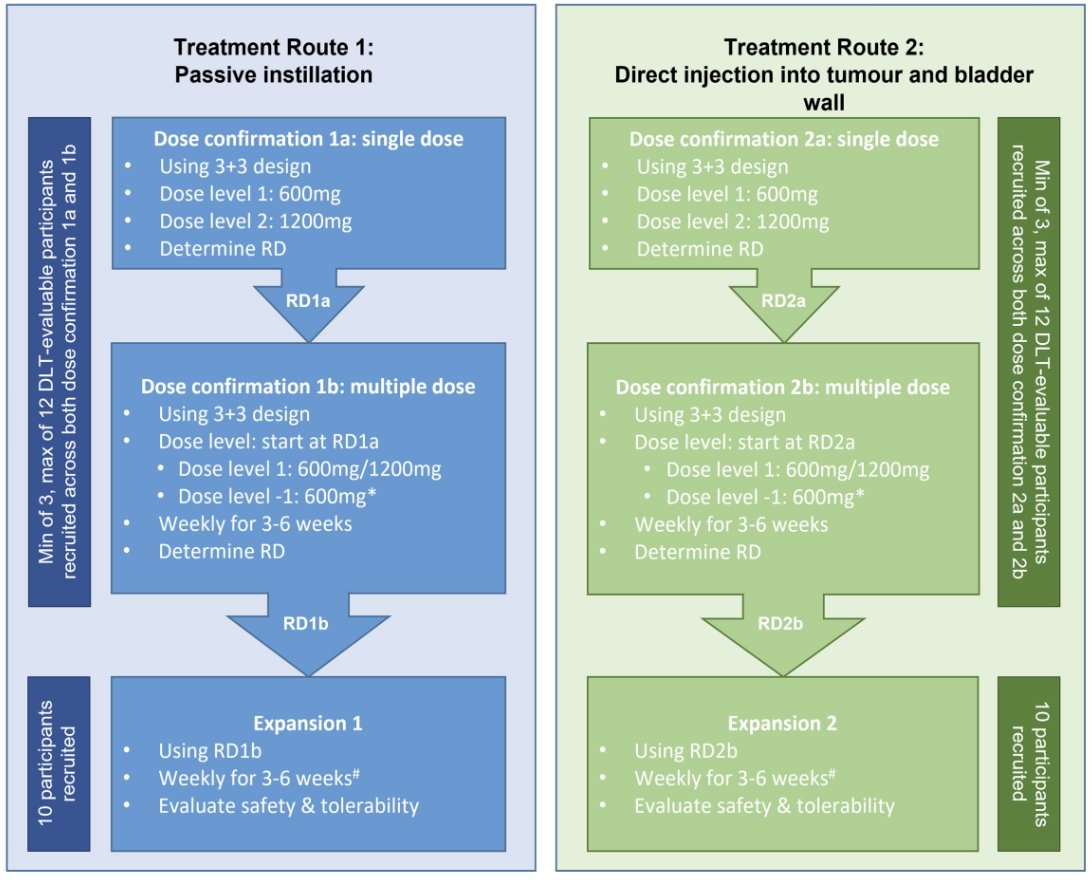
Efficacy signals will be explored via progression-free survival at two years and pathological complete response rate. INVEST will also assess the pharmacokinetic profile of intravesical atezolizumab, anti-drug antibodies to atezolizumab. Samples are being prospectively collected for future translational research. Sampling (fresh, frozen, and FFPE blocks) of the scar, tumor and background urothelium is being performed on cystectomy specimens, and tissue microarrays will be created from TURBT and cystectomy tissue. Translational samples include pre- and post-treatment blood cfDNA, PBMCs and serum and urine cfDNA. The trial will also assess drug penetration into the bladder wall and the effects of treatment on circulating tumor cells, and local and systemic immune cell composition.
Enrolment began in May 2023, with a planned 2-year recruitment period and 2-year follow-up period at a single UK site. To date, six patients have been recruited and treated: 4 treatment route 1 dose confirmation 1a (3 at dose level 1, 1 at dose level 2), and 2 at treatment route 2 dose confirmation 2a (dose level 1). There have been no dose limiting toxicities to date.
Clinical trial information: ISRCTN15842444.
Presented by: Syed A. Hussain, MBBS, MSc, MD, FRCP, University of Sheffield and Sheffield Teaching Hospitals, Sheffield, UK
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicenter, phase 2 study. Lancet Oncol. 2021 Jul;22(7):919-930.


