(UroToday.com) Alterations in the Androgen Receptor (AR) remain a common locus for castration resistance in prostate cancer (CRPC), and impact response to AR-targeted novel hormonal therapies, including enzalutamide. Dr. Laccetti and colleagues present preliminary safety and efficacy data on the combination of enzalutamide with a next generation antigen EPI-7386 in chemotherapy-exposed patients with CRPC prior to exposure to any AR pathway inhibitor.
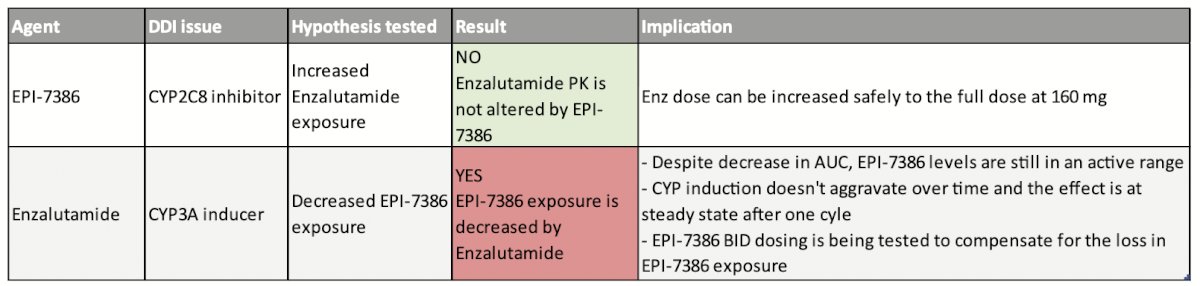
EPI-7386 mechanism of action is described in the poster as through binding to the N-terminal domain (NTD) of AR and thereby blocking transcription without respect to any resistance-driving mechanisms in the ligand-binding domain (LBD). The authors describe preclinical RNAseq and CHIPseq data indicating that combination of EPI-7386 with enzalutamide results in deep blockade of AR pathway and with greater antitumor activity than with enzalutamide alone. This rationale led to the development of this phase 1/2 multicenter, open-label clinical trial (NCT05075577). Eligible patients include those with metastatic CRPC on ADT and without prior exposure to second-generation antiandrogens. One prior line of chemotherapy is permitted. In the Phase I portion, escalating doses of EPI-7386 are tested in combination with a fixed dose of enzalutamide. Primary and secondary endpoints are safety and pharmacokinetics (PK) with the goal of determining the recommended Phase 2 combination doses (RP2CDs). This is particularly important given that EPI-7386 is a known inhibitor of CYP2C8, a metabolizer of enzalutamide, and thus potentially increasing patient exposure to enzalutamide. Conversely, enzalutamide is a known inducer of CYP enzymes that participate in metabolism of EPI-7386, thereby potentially decreasing patient exposure to the novel drug.
Here the authors present data from the first 7 patients treated, including from Cohort 1 (n=3, EPI-7386 600 mg QD + enzalutamide 120 mg QD) and Cohort 2 (n=4, EPI-7386 800 mg QD + enzalutamide 120 mg QD). No DLTs have been observed and the safety profile was considered consistent with second-generation antiandrogen, including grade 1-2 fatigue and hot flashes.
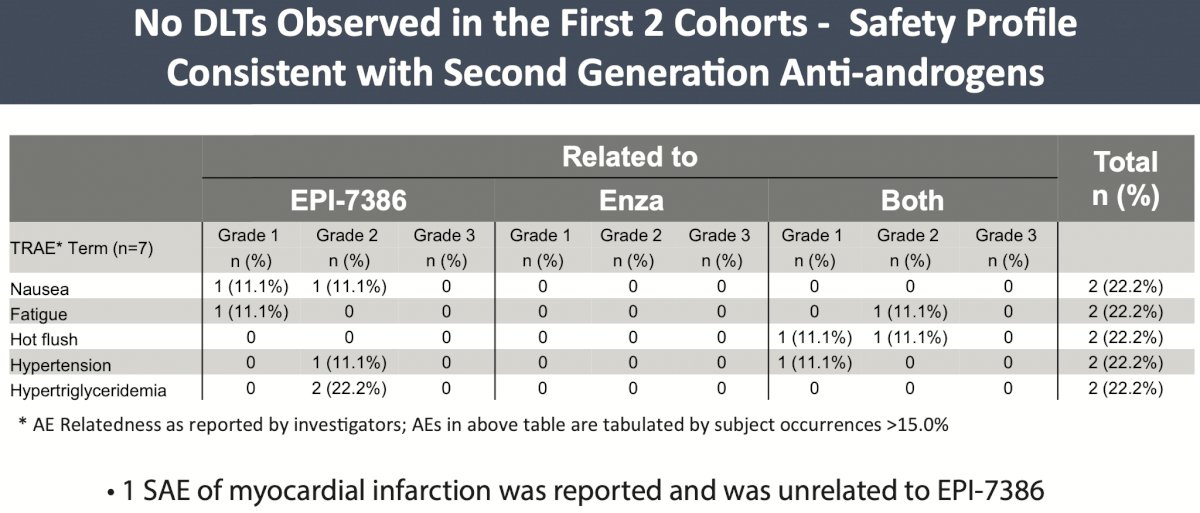
The authors also shared some preliminary efficacy data for these 6 of 7 patients. The patient deemed non-evaluable for efficacy after discontinuation after cycle 1 due to a strong CYP3A4 inducer concomitant medication which lowered exposure to both EPI-7386 and enzalutamide. Among the remaining 6, 5 experienced a PSA decline of at least 90% (PSA90) with 4 attaining a PSA of <0.2 ng/mL. All 6 of these patients demonstrated stable imaging

The authors offered as historical comparisons data from the PREVAIL, PREMISE, and AFFIRM studies, and concluded that PSA responses to the combination of EPI-7386 and enzalutamide compare favorably with these studies of enzalutamide + ADT alone. Overall conclusions were clearly summarized by Dr. Laccetti’s poster including (1) combination of EP-7386 with enzalutamide was safe and well-tolerated, with reduction of EPI-7386 by enzalutamide (likely via CYP3A4), and without significant impact on enzalutamide by EPO-7386 and (2) rapid and deep PSA responses were observed in 5/6 patients. They describe dose escalation to cohort 3 (EPI-7386 600 mg BID + enzalutamide 120 mg QD) which is fully enrolled and awaiting DLT period completion. Pending successful safe completion, the Phase 2 portion of the study will compare combination dosing to single agent enzalutamide at full standard of care dose (160 mg QD) in a 2:1 randomization in the same patient population.
Presented by: Andrew Laccetti, MD, MS, Memorial Sloan Kettering Cancer Center, New York, NY
Written by: Jones Nauseef, MD, PhD, Assistant Professor of Medicine within the Division of Hematology and Medical Oncology, Sandra and Edward Meyer Cancer Center, and Englander Institute for Precision Medicine Weill Cornell Medicine and Assistant Attending physician at NewYork-Presbyterian Hospital. @DrJonesNauseef on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
View the Video Discussion with presenting author Andrew Laccetti:
Phase 1/2 Study of EPI-7386 in Combination with Enzalutamide in Metastatic Castration-Resistant Prostate Cancer- Andrew Laccetti


