The 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between February 16th and 18th was host to a prostate cancer poster session. Dr. Joseph Thomas presented his group’s study evaluating the increased utilization of prostate-specific membrane antigen (PSMA)-targeted radionuclide therapy (PSMA-TRT) in African American (AA) patients at their Academic Medical Center.
Dr. Thomas began by highlighting that Weill Cornell Medicine (WMC) has had a research program utilizing anti-PSMA monoclonal antibody (J591) since the year 2000. Furthermore, since the emergence of PSMA ligand-based therapies in 2017, WCM has enrolled around 300 patients on investigational PSMA-TRT clinical trials. Since the approval of 177Lu-PSMA-617 (Pluvicto; Lu-177 vipivotide tetraxetan), WBC began standard-of-care (SOC) treatment, co-enrolling willing patients into the research registry. As such, this registry provides a unique opportunity to chart enrolment and outcomes trends since early 2000.
It is well-recognized that African American patients are disparately less likely to be enrolled in clinical trials of therapeutic agents in the United States.1 As such, WCM has made a concerted effort to increase the number of African American prostate cancer patients enrolling in clinical trials at WCM. In this analysis, the authors retrospectively evaluated the demographic data of patients enrolled on PSMA-TRT clinical trials to determine whether there have been any significant changes in enrollment trends and to assess whether or not efforts to improve access to this novel treatment for this historically under-represented, high risk cohort of patients.
The authors collected demographic (i.e. racial/ethnic) data for WMC patients with prostate cancer, who were included in the PSMA-TRT investigational clinical trial database or who were enrolled on the WCM SOC 177Lu-PSMA-617 research registry. The authors used self-reported race and , when not available (due to patient death or loss of follow-up), demographic information documented in the medical record. Patients were assigned to five-year cohorts based on either date of consent for trial enrollment or the start date of PSMA-TRT treatment (depending on available information). The institutional tumor registry data was used as a reference comparator to assess the total percentage of African American patients with prostate cancer seen at WCM.
The percentage of African American patients enrolled in PSMA-TRT clinic trials at WC were as follows:
- 2000 to 2004: 3.1% (2/65)
- 2005 to 2009: 5.1% (3/59)
- 2010 to 2014: 6.1% (2/33)
- 2015 to 2019: 5.9% (5/85)
- 2020 to July 2022: 11.1% (8/72)
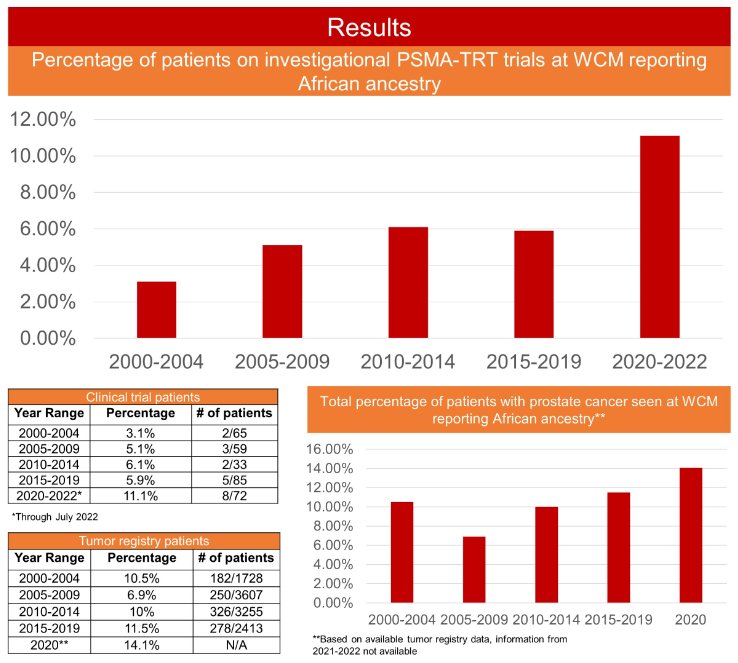
In comparison, the total percentages of African American patients in the WCM overall tumor registry data were as follows:
- 2000 to 2004: 10.5% (182/1,728)
- 2005 to 2009: 6.9% (250/3,607)
- 2010 to 2014: 10% (326/3255)
- 2015 to 2019: 11.5% (278/2,413)
- 2020: 14.1% (Not available for 2021 and beyond).
The authors concluded that the percentage of African American patients on investigational PSMA-TRT trials at WCM significantly increased between 2000 and 2019 (3.1% to 6.1%), with even higher rates of 11.% in 2020 to 2022. Significantly, 50% of those treated with 177Lu-PSMA-617 since its FDA approval and co-enrolled in the WCM research registry identified as African American. These data suggest that outreach and increasing access to African American patients for novel prostate cancer treatment such as PSMA-TRT may be causally associated with increased numbers of underrepresented patients enrolling on clinical trials and gaining access to novel medical therapeutic regimens.
Presented by: Joseph Earl Thomas, MD, Hematology-Oncology Fellow, Department of Medicine, New York-Presbyterian/Weill Cornell Medical Center, New York City, NY
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, February 16th – February 18th, 2023
References:


