(UroToday.com) The 2023 GU ASCO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Neal Shore discussing long-term safety and tolerability of darolutamide and duration of treatment in patients with nonmetastatic castration-resistant prostate cancer (nmCRPC) from the ARAMIS Rollover Study. Darolutamide significantly improved metastasis-free survival by ~2 years and reduced the risk of death by 31% vs placebo, with a favorable safety profile in patients with nmCRPC (ARAMIS study;1 NCT02200614). At the 2023 GU ASCO meeting, Dr. Shore and colleagues reported long-term safety and tolerability with continued darolutamide treatment in the ARAMIS Rollover Study (NCT04464226).
Following the primary analysis of double-blind treatment, the ARAMIS study was unblinded, and all patients were permitted to continue on open-label darolutamide. After study completion, patients could continue darolutamide in the rollover study if they had no evidence of metastases and were benefitting clinically. Of 955 patients initially randomized to darolutamide, 954 patients started double-blind darolutamide, 466 continued to open-label darolutamide, and 294 entered in the rollover study and received darolutamide 600 mg orally twice daily. Darolutamide safety and treatment duration are described for the 954 patients over the double-blind, double-blind + open-label, and double-blind + open-label + rollover study periods, with a data cutoff of January 31, 2022. The trial design for this study is as follows:

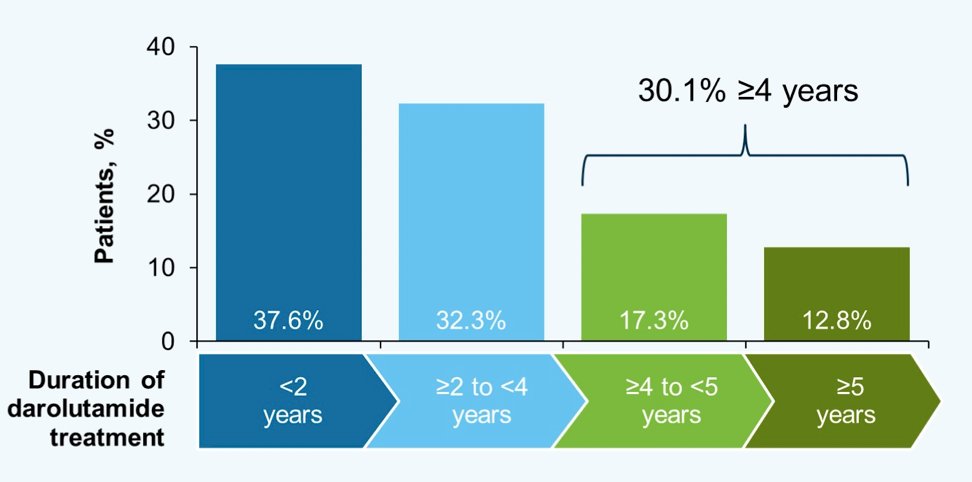
The median treatment duration was 1.5 years (range 0.0–4.0) for double-blind darolutamide, 2.1 years (range 0.0–4.9) for double-blind + open-label darolutamide, and 2.8 years (range 0.0–6.8) for double-blind + open-label + rollover study darolutamide. By the data cutoff date, 62.4% of the 954 patients had received darolutamide for ≥2 years, 30.1% for ≥4 years, and 12.8% for ≥5 yrs. There were 24.0% of men still receiving darolutamide at data cutoff:
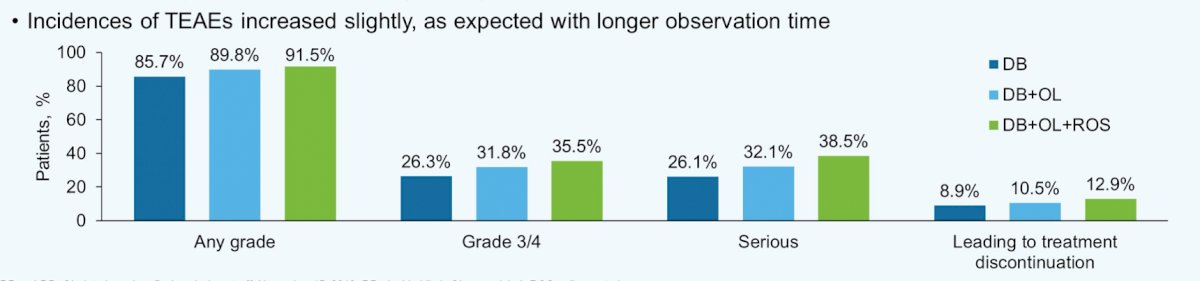
Incidences of treatment-emergent adverse events increased slightly as expected with longer observation time:
- Any grade (double-blind 85.7%, double-blind + open-label 89.8%, double-blind + open-label + rollover study 91.5%)
- Grade 3/4 (double-blind 26.3%, double-blind + open-label 31.8%, double-blind + open-label + rollover study 35.5%)
- Serious (double-blind 26.1%, double-blind + open-label 32.1%, double-blind + open-label + rollover study 38.5%)
- Leading to treatment discontinuation (double-blind 8.9%, double-blind + open-label 10.5%, double-blind + open-label + rollover study 12.9%)
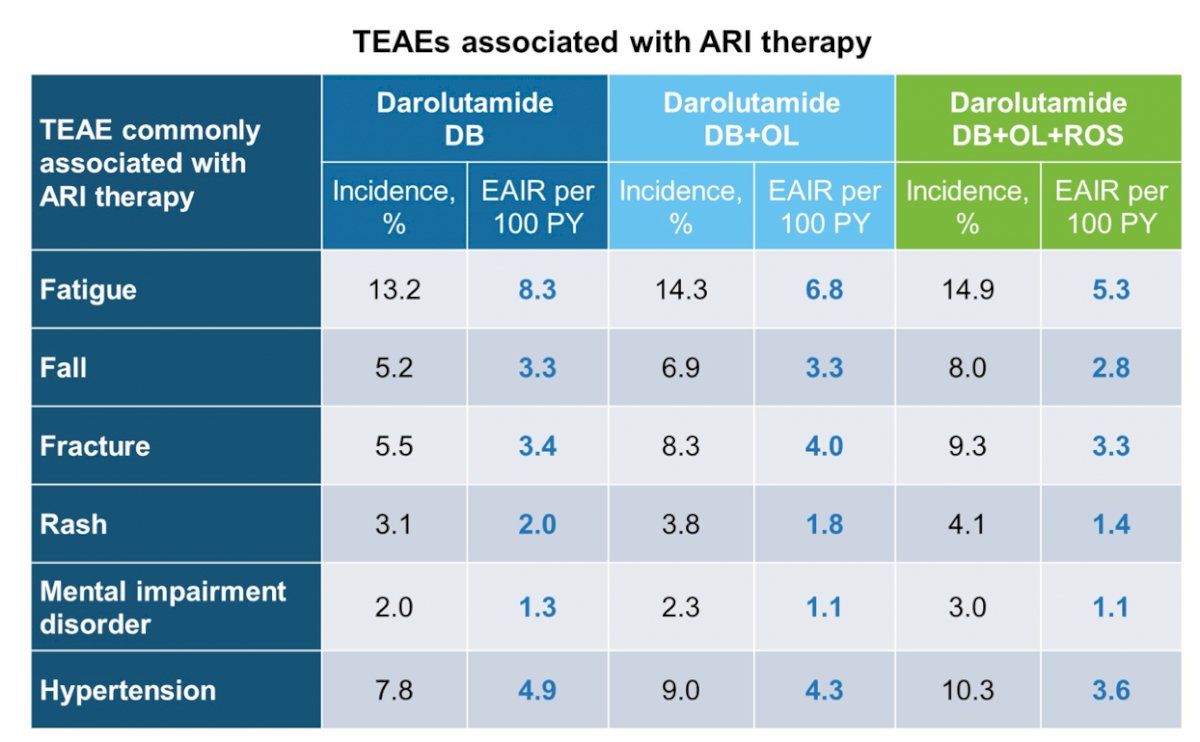
Increases in incidences of treatment-emergent adverse events of special interest across the double-blind, double-blind + open-label, and double-blind + open-label + rollover study periods were mostly minimal:
Dr. Shore concluded his presentation discussing long-term safety and tolerability of darolutamide and duration of treatment in patients with nmCRPC from the ARAMIS Rollover Study with the following concluding messages:
- The ARAMIS Rollover Study allowed the duration of darolutamide treatment and the long-term safety of darolutamide to be evaluated in patients with nmCRPC
- Approximately 30% of patients with nmCRPC remained on darolutamide for ≥4 years, suggesting long-term clinical benefit
- The favorable safety profile of darolutamide was maintained with long-term exposure
- No new safety concerns were observed with longer darolutamide treatment in the rollover study
- These findings indicate that the clinical benefit and favorable safety profile of darolutamide in nmCRPC are maintained for several years in some patients
Presented by: Neal D. Shore, MD, FACS, Medical Director, Carolina Urologic Research Center, Atlantic Urology Clinics, Myrtle Beach, SC
Co-Authors: Murilo de Almeida Luz, Albertas Ulys, Jorge A. Ortiz, Shankar Srinivasan, Etah Kurland, Karim Fizazi
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
References:
- Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246.


