(UroToday.com) On the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2023 focussing on prostate cancer, Dr. Arun Azad presented data examining the efficacy of subsequent systemic therapies after Lu177 radioligand therapy for patients with metastatic castration-resistant prostate cancer (mCRPC) in Poster Session A.
Based on data from the phase III VISION trial, [177Lu]Lu-PSMA (LuPSMA) is currently FDA-approved for use in the post-taxane, post-antiandrogen setting in patients with mCRPC. However, as this trial assessed patients who had received nearly all available therapies prior to enrollment, little is known about the efficacy of treatment in patients with subsequent progression after LuPSMA. To better address this knowledge gap, the authors performed a single-centre retrospective analysis to evaluate the efficacy of first subsequent therapy following LuPSMA.
The identified 234 patients with mCRPC who received LuPSMA at the Peter MacCallum Cancer Centre from 2015-2022. Among these 234 men, 136 were excluded as they did not receive any subsequent treatment (n=136) and an addition 25 were excluded as they either received the initial course of LuPSMA in combination with another therapy (n=10) or had insufficient follow-up data (n=15). Retreatment withLuPSMA was considered a subsequent line of therapy. The authors examined 50% PSA response rate (PSA-RR), PSA-progression free survival (PSA-PFS) and overall survival (OS) and calculated survival outcomes using Kaplan-Meier analysis.
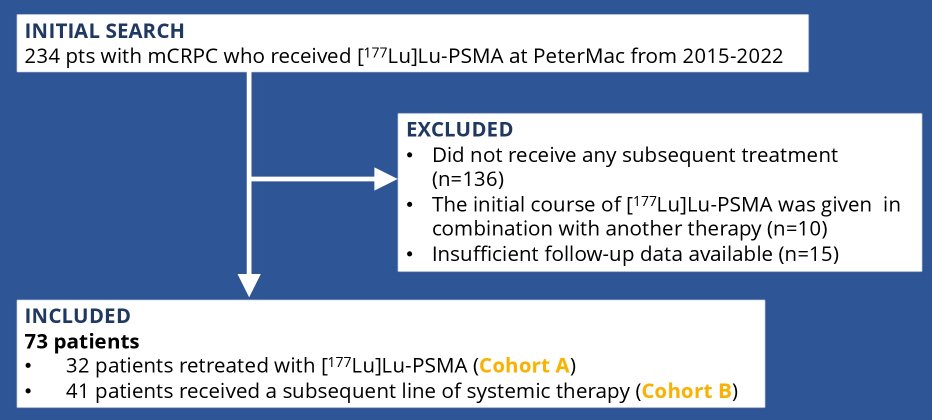
Among the 73 evaluable patients, 32 were retreated with LuPSMA (median 2 cycles) and 41 commenced a new line of systemic therapy. Among those in the LuPSMA retreatment group, the PSA-RR was 44%, median PSA-PFS was 3.8 months and median OS 14.8 months. In patients who changed to a new line of treatment, cabazitaxel was the most commonly prescribed therapy (n=25), followed by docetaxel (n=7), and single-agent carboplatin (n=4), in addition to carboplatin/etoposide, mitoxantrone, olaparib, capecitabine and a clinical trial (n=1 for each). PSA-RR was 12%, median PSA-PFS was 3.5 months and median OS 6.6 months.
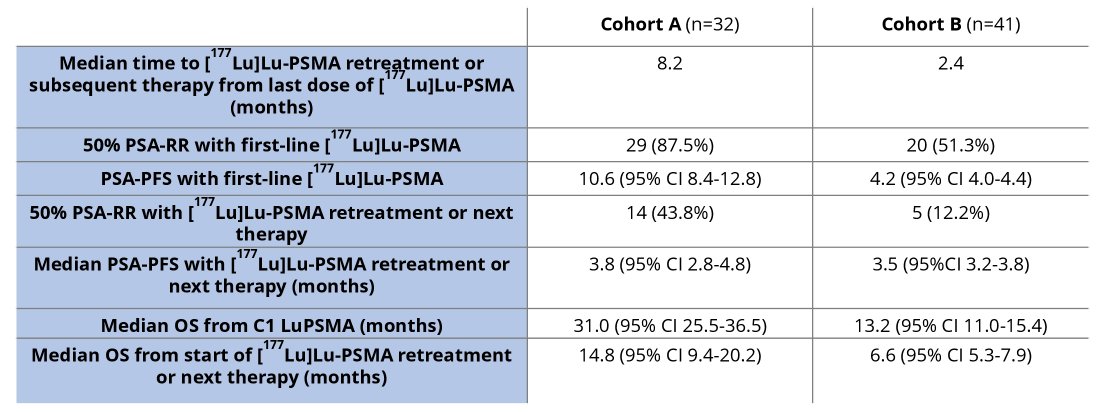
Thus, these authors conclude that these retrospective data demonstrates that retreatment with LuPSMA is associated with benefit, though this is reduced compared to initial treatment. However, most patients were not deemed suitable for retreatment with LuPSMA, potentially reflecting de-differentiated and more aggressive disease phenotypes. In those not suitable for LuPSMA retreatment, other systemic therapy appears to have limited benefit, highlighting the lack of effective salvage options and raising questions about treatment sequencing.
Presented by: Arun Azad, PhD, MBBS, FRACP | Peter MacCallum Cancer Centre; and Sir Peter MacCallum Department of Oncology, University of Melbourne

