(UroToday.com) The 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between February 16th and 18th was host to a Renal Cell Cancer; Adrenal, Penile, Urethral and Testicular Cancers poster session. Dr. Thomas Powles presented the outcomes by IMDC risk in the COSMIC-313 phase 3 trial evaluating cabozantinib plus nivolumab and ipilimumab in first-line advanced RCC of IMDC intermediate or poor risk.
COSMIC-313 is a global, randomized, double-blind, phase 3 trial that evaluated the triplet regimen of cabozantinib + nivolumab + ipilimumab versus the control regimen of placebo + nivolumab + ipilimumab in previously untreated patients with advanced renal cell carcinoma (RCC) of International Metastatic RCC Database Consortium (IMDC) intermediate or poor risk. This trial was the first study to use a standard of care immune-oncology doublet as a control.
In the primary analysis of progression-free survival (PFS) in the PFS intention-to-treat (PITT) population, the triplet regimen significantly improved PFS compared with the control:
- Median PFS was not reached versus 11.3 months, respectively (HR: 0.73, 95% CI: 0.57 – 0.94, p=0.013) after a median follow-up of 14.9 months
Subgroup analyses suggested that the PFS and objective response rate (ORR) benefit with the triplet regimen versus control was primarily in IMDC intermediate risk patients. In this report, the authors presented updated PFS results, as well as outcomes and exposure by IMDC risk group.
The ITT population included a total of 855 patients who were randomized 1:1 to the triplet or control regimens.
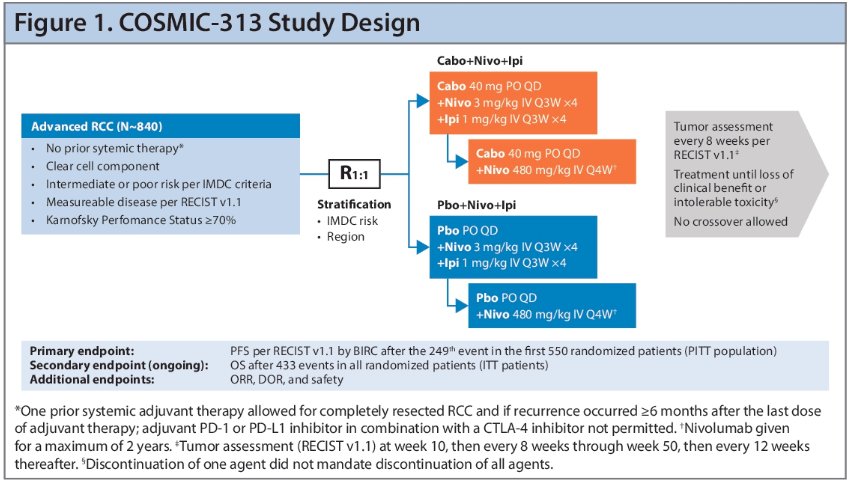
The primary endpoint was PFS per RECIST v1.1 by blinded independent central review (BICR) after the 249th PFS events in the first 550 randomized patients (PITT population), which occurred on August 23, 2021. The updated analysis reported here occurred at a data cutoff of January 31, 2022, after 266 PFS events in the PITT population and 402 events in the ITT population had occurred. The secondary endpoint is OS, with final analysis after 433 events in all randomized patients. Patients continue to be followed for OS under blinded conditions, with results pending. Additional endpoints of interest include:
- ORR
- Duration of response
- Safety and adverse events, reported according to National Cancer Institute Common Terminology Criteria for Adverse Events v5.0
Overall, 75% of patients were IMDC intermediate risk and 25% were poor risk. Patients with intermediate risk disease had better Karnofsky Performance Status at baseline (≥90: 66% versus 47% for poor risk) and were more likely to have had a prior nephrectomy (71% versus 45%).
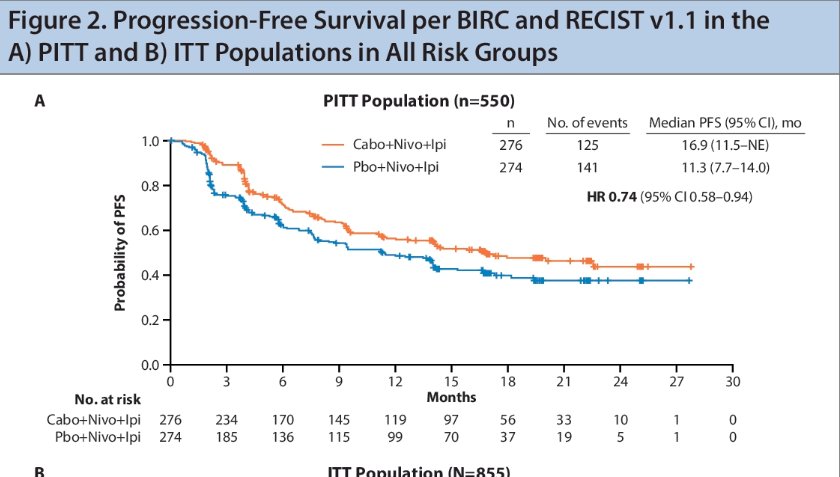
In the overall patient population, the PFS benefit observed in the primary analysis was maintained after ~5 additional months of follow up.
- In the PITT population, a 26% reduction in the risk of progression or death was observed with the triplet regimen versus the control (median PFS: 16.9 versus 11.3 months; HR: 0.74), after 20.2 months median follow up.
-A similar result was observed in the ITT population with a median PFS of 15.3 months for the triplet regimen and 11.3 months with the control (HR: 0.74), after 17.7 months median follow-up
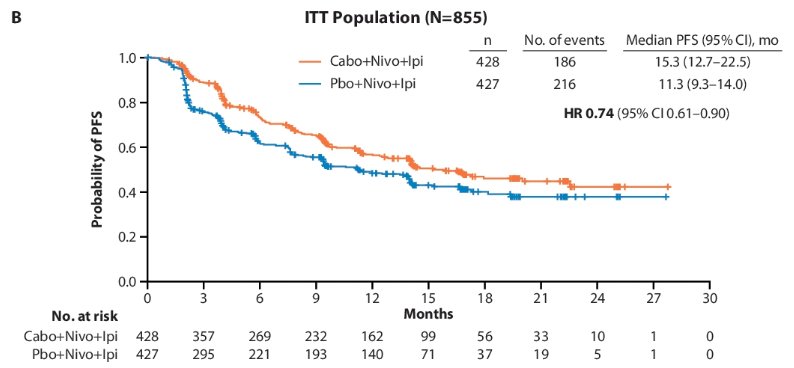
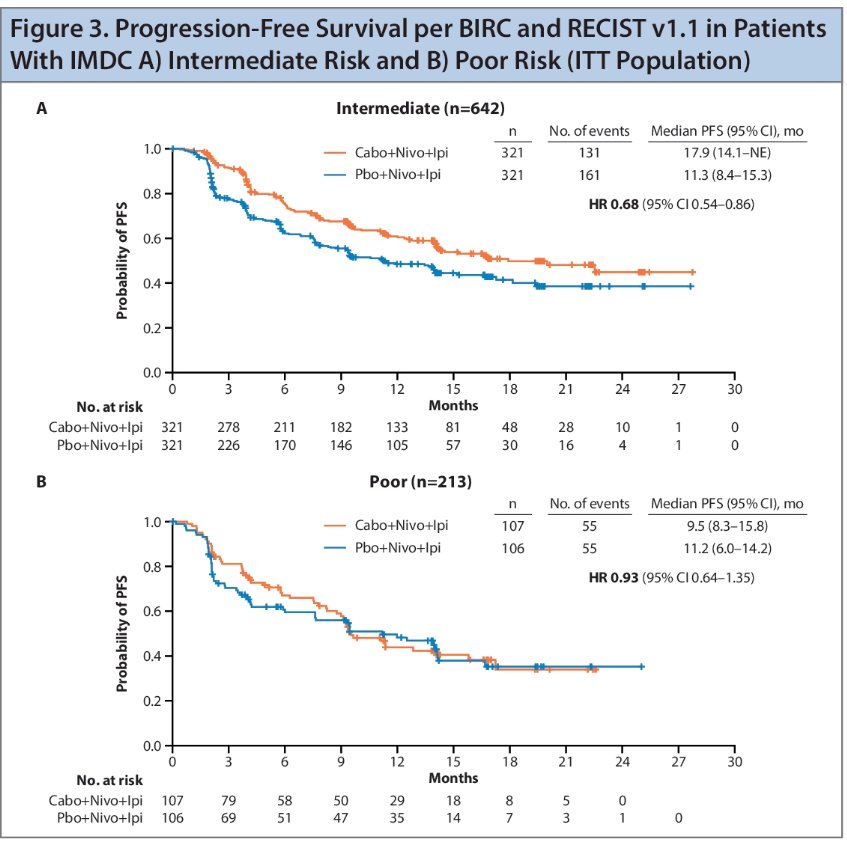
In patients with intermediate-risk disease in the ITT population, a 32% reduction in the risk of progression or death was observed with a median PFS of 17.9 months for the triplet versus 11.3 months for the control (HR: 0.68). Among patients with poor risk disease, median PFS was 9.5 months versus 11.2 months, respectively.
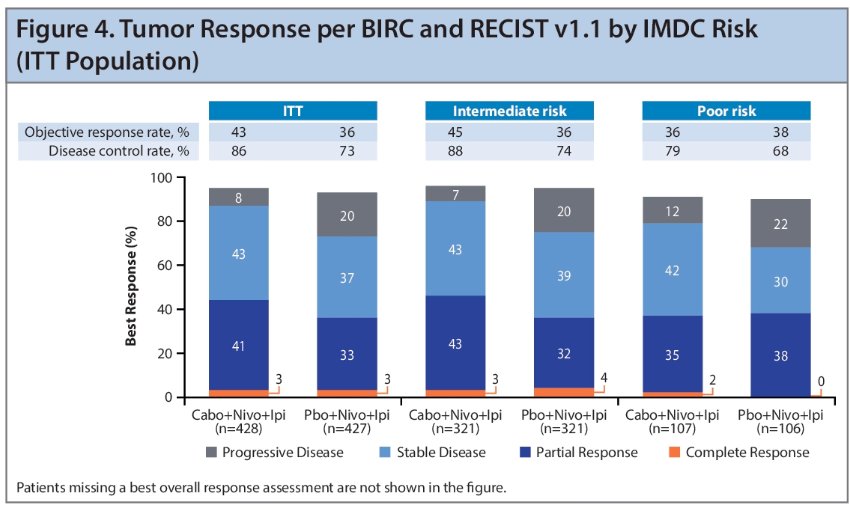
The ORR was higher with the triplet regimen versus control in intermediate-risk patients, but not for poor-risk patients. The triplet regimen demonstrated a higher DCR, and a lower rate of PD as best response compared with the control in both risk groups.
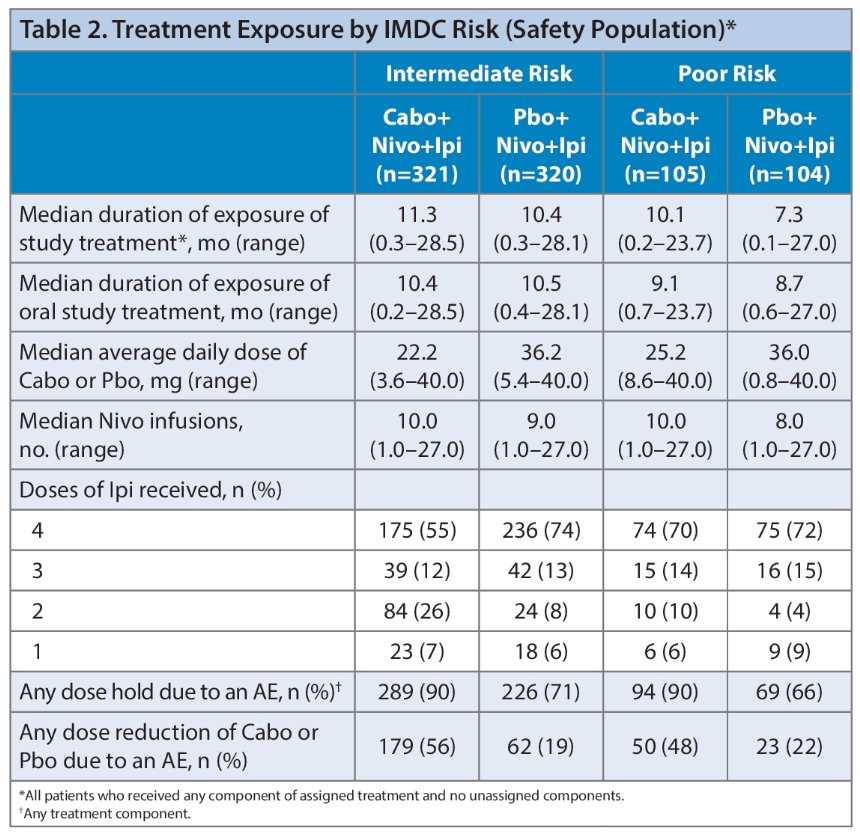
There were no major differences in exposure between treatment arms in either risk group that could explain the observed differences in efficacy between intermediate- and poor-risk groups.
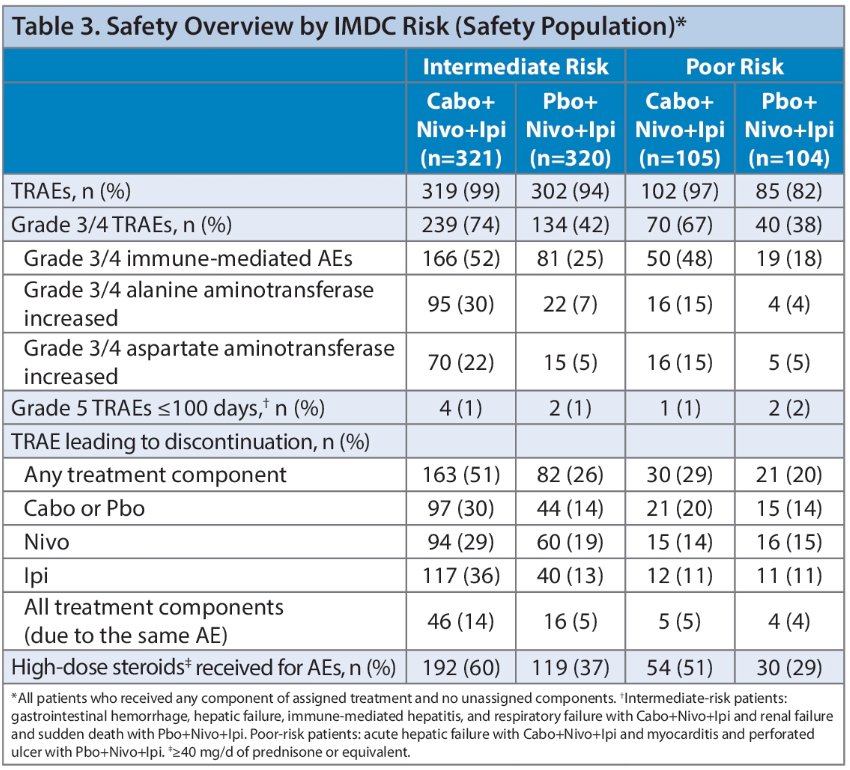
Among patients randomized to the triplet regimen, treatment-related adverse events (TRAEs) led to discontinuation of treatment components in a greater proportion of intermediate-risk compared with poor-risk patients.
Dr. Powles concluded as follows:
- Updated analysis of COSMIC-313 remains consistent with the primary analysis. The PFS benefit with the triplet regimen was maintained over an additional follow up of ~5 months in the overall population (HR: 0.74), as well as in intermediate-risk patients (HR: 0.68)
- There were no major differences in exposure that could explain the differences in efficacy between intermediate- and poor-risk groups for the triplet regimen
- AEs led to treatment discontinuation more frequently in intermediate-risk group compared with poor-risk group for the triplet regimen
- Follow-up for OS is ongoing
Presented by: Thomas Powles, MBBS, MRCP, MD, Professor of Genitourinary Oncology, Director, Barts Cancer Centre at St. Bartholomew's Hospital, London, UK
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
References:- Choueiri TK, et al. Ann Oncol. 2022;33(suppl_7):S1430-S1431.


