(UroToday.com) The 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between February 16th and 18th was host to a prostate cancer and urothelial carcinoma poster session. Olivia Pocha presented the preliminary results of the FIREFLY study, evaluating 68Ga-PSMA-11 in patients with newly diagnosed and recurrent prostate cancer.
Prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) has been demonstrated to have superior sensitivity and specificity compared to conventional imaging.1 However, a major challenge in the United States remained access to PSMA PET/CT imaging prior to the recent FDA approval. The FIREFLY study was designed to offer PSMA imaging to patients between May 2021 and May 2022 to better characterize their disease. This study was designed to accrue 300 patients but was prematurely terminated when Illucix® imaging became commercially available.
This was a phase II expanded access clinical trial under Tulane’s own Investigational New Drug application with the FDA for 68Ga-PSMA-11 (Telix Pharma cold kit, NCT: 04854369). Patients received 1.8-2.2 MBq/kg body weight of 68Ga-PSMA-11. The lower and upper dose limits were set to 3 and 7 mCI, respectively.
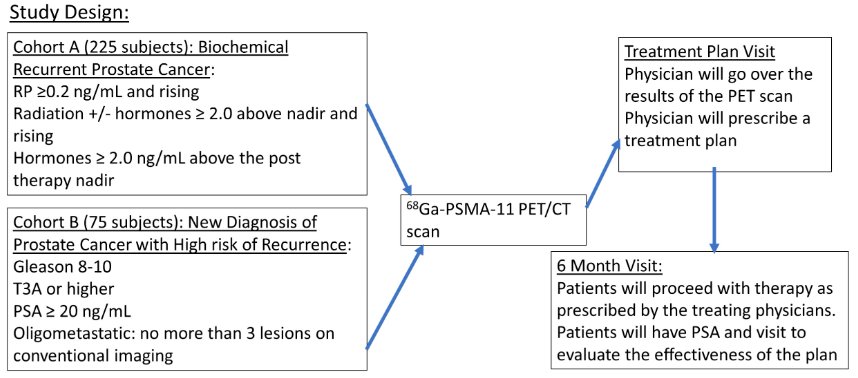
The study included 2 cohorts:
- Cohort A (n=225): Biochemical failure
- Post-radical prostatectomy: ≥0.2 ng/ml and rising
- Radiation +/- ADT: ≥2.0 ng/ml above nadir and rising
- ADT: ≥2.0 ng/ml above nadir
- Cohort B (n=75): High recurrence risk, pre-treatment
- Gleason Score 8-10
- T3a or worse
- PSA ≥20 ng/ml
- Oligometastatic: no more than 3 lesions on conventional imaging
The study design was as follows:
The primary objective was:
- Utilization of 68Ga-PSMA-11 PET images to define uptake location for localization of metastatic sites in patients prior to initial therapy or after recurrence
Secondary objectives included:
- Assess the therapeutic consequences of 68Ga-PSMA-11 PET/CT imaging in prostate cancer with and without prior treatment
- To determine the number of patients with metastatic disease prior to initial therapy absent on conventional scans
- To determine locations of recurrent disease for those patients who have PSA recurrence after curative intent therapy
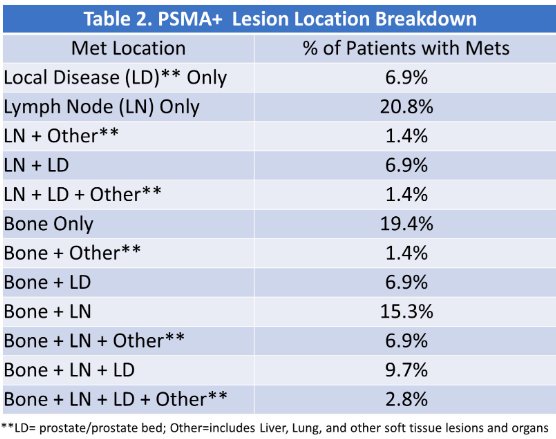
This study was prematurely terminated with 80/225 patients recruited to Cohort A and 9/75 patients in cohort B. Of the 89 patients, 70 (79%) had PSMA positive lesions.
The most common sites of PSMA positive sites were lymph nodes only (21%), bone only (19.4%), and bone + lymph nodes (15.3%):
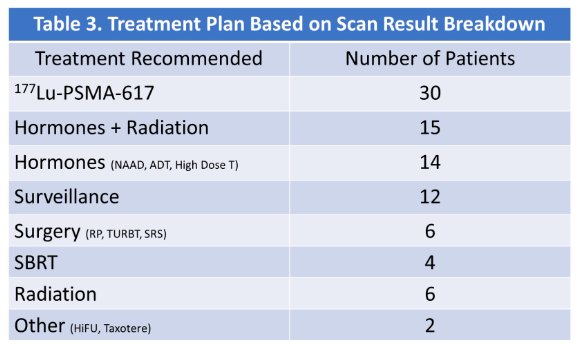
30/89 patients subsequently received 177Lu-PSMA-617 therapy, with hormones + XRT the 2nd most common treatment modality:
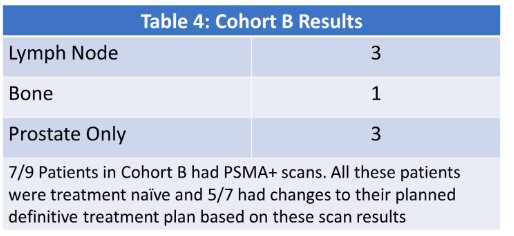
Of the 9 patients in cohort B, 7 (77.7%) had PSMA+ scans. All these patients were treatment naïve and 5/7 had changes to their planned definitive treatment plan based on these scan results:
The investigators concluded that PSMA PET/CT allows physicians to more accurately stage patients, and their findings were consistent to those reported in the literature. In their opinion, the FIREFLY study allowed investigators to make better informed decisions and create patients-specific treatment plans.
Presented by: Olivia Pocha, Study Coordinator, Tulane Cancer Center, New Orleans, LA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, February 16th – February 18th, 2023
Reference:
- Hofman MS, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 2020;395(10231):1208-1216.


