(UroToday.com) On the second day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2023 focussing on urothelial cancer, Dr. Michiel Simon Van Der Heijden provided an invited discussants perspective following two oral presentations in the Oral Abstract Session B: from Dr. Matt Galsky highlighting updated data from the CheckMate 274 trial with extended follow-up results examining adjuvant nivolumab for patients with resected muscle-invasive urothelial carcinoma and from Dr. Andrea Neechi discussing results of pembrolizumab monotherapy in patients with high-risk non–muscle-invasive bladder cancer (HR NMIBC) unresponsive to bacillus Calmette–Guérin (BCG) in the context of cohort B of the phase 2 KEYNOTE-057 trial.
Dr. Van Der Heijden, in alluding to the theme of his talk, subtitled it “Does the end justify the means?”. This is certainly a relevant question in this disease space as, for patients with high risk non-muscle invasive bladder cancer (HR NMIBC), patients are not imminently at risk of dying of disease and may be relatively effectively cured with cystectomy. Thus, goals for systemic therapy in this population are different than in many systemic therapy indications, notably, to substantially delay the time of cystectomy with a relatively low rate of progression to muscle invasive disease. As a result, the endpoints for this trial also differ. Based on work from the International Bladder Cancer Group led by Dr. Kamat, he noted that it is accepted that a reasonable target is >30% freedom from high-risk recurrence at 1 year among patients with HR NMIBC who have papillary disease.
In contextualizing this endpoint, he noted that there is considerable heterogeneity in this disease cohort, including in terms of tumor stage, tumor multifocality, tumor size, the interval from last BCG treatment, and other characteristics. Thus, he emphasized that one of the keys in managing patients with HR NMIBC is identifying those likely to have aggressive disease biology despite relatively benign appearances, the so-called wolf in sheep’s clothing.
Dr. Van Der Heijden then highlighted the KEYNOTE-057 study schema emphasizing that disease assessment was by cystoscopy and cytology, without mandated biopsy. He thinks it's probably fine in cohort B, among patients, initially presenting with papillary disease, though it would be less so among a cohort of patients with CIS.

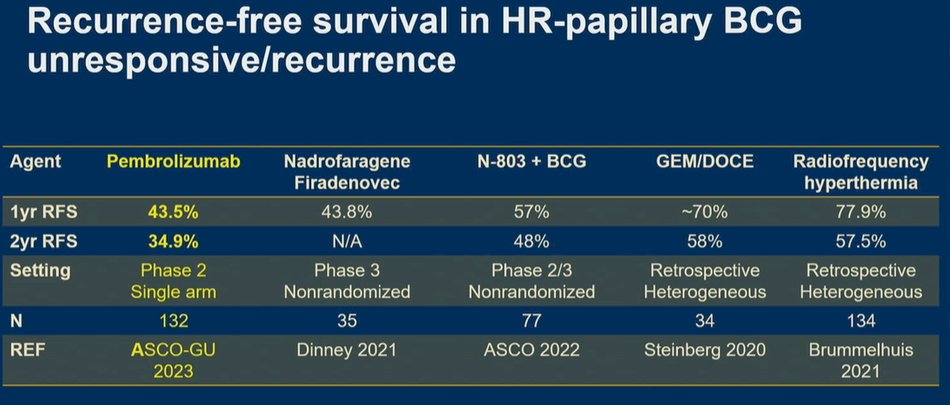
After highlighting results from KEYNOTE-057 showing a disease-free survival of 43.5% at one year, he noted that other treatment approaches in this disease space show relatively comparable results. To this end, he highlighted subgroup data among patients with high grade Ta and T1 disease treated with nadofaragene firadenovec showing nearly identical 1 year rates (43.8%). While not accrued in similarly rigorous prospective trials, results are relatively comparable for radio-frequency induced hyperthermia with intravesical chemotherapy and for gemcitabine with docetaxel. Finally, preliminary data from QUILT-3032-Cohort B suggest even better results with N-803 plus BCG. He noted that the data for pembrolizumab from KEYNOTE-057 stand out due to the larger sample size and longer durations of follow-up.
In spite of this, he admitted to being personally “a bit hesitant” to embrace this concept. In part, he noted that this was due to personal experience from neoadjuvant trials of immunotherapy prior to cystectomy. Additionally, he cited that both Ta and CIS disease are within the epithelial basement membrane and there may be lower penetrance of antibodies. Further, NMIBC has much lower tumor mutational burden than muscle invasive disease. Thus, while this was, in his words, a well-conducted phase 2 study with (probable) clinical activity, he noted that the magnitude of benefit was uncertain. As a result, he advocated for randomized trials, though acknowledging the difficulty in identifying the appropriate control arm in such a randomized study.
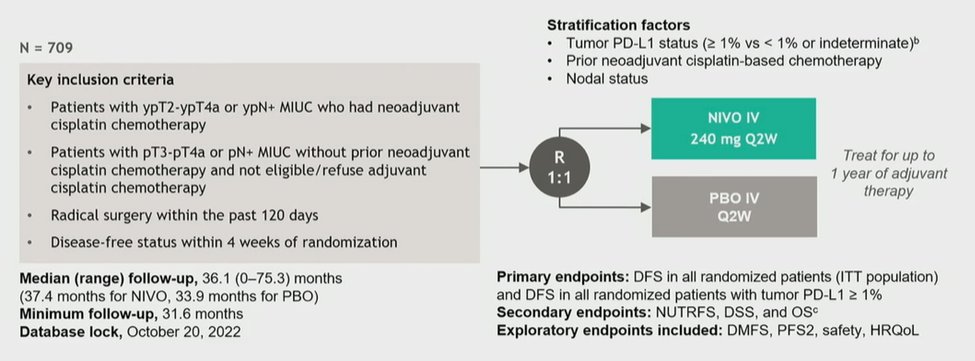
Dr. Van Der Heijden then moved to discussing the abstract from Dr. Galsky and colleagues, with extended follow-up of the CheckMate 274 trial assessing adjuvant nivolumab following resection of muscle invasive urothelial disease.
He noted that, in the adjuvant setting, we need to be clear of the goals of therapy. This is particularly important as some patients will be cured with resection alone and we need to consider if we can justify the overtreatment of these patients. Further, he noted that a DFS benefit doesn’t inherently confer a higher cure rate as it may simply prolong the period before identification of radiographically visible disease.

He noted that, in the past, there has been a “problematic” relationship with adjuvant therapy in muscle invasive bladder cancer, though in the context of chemotherapy. As reported by Dr. Sternberg, the EORTC 30994 trial showed a dramatic diminishment of benefit in terms of overall survival compared to progression-free survival benefit, in spite of a lack of efficacious subsequent therapies at that time.

Further, in the immunotherapy space, the IMvigor010 trial showed no benefit in either endpoint for the use of adjuvant atezolizumab in the same clinical circumstance. In spite of this, the data from CheckMate 274, both at initial publication and in these updated data, demonstrate a consistent benefit for DFS in both the ITT and PDL1-positive populations, with a somewhat larger magnitude of effect in the PDL1-positive population.

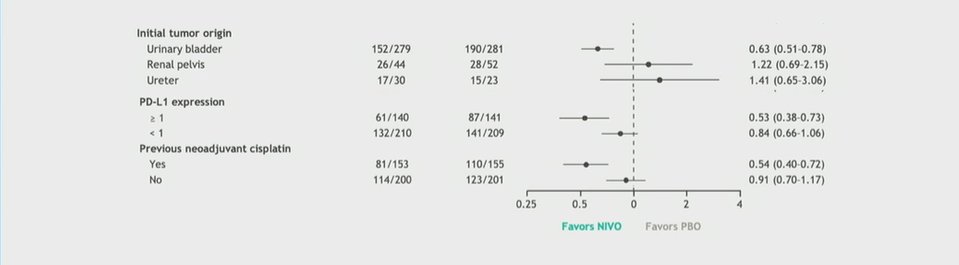
Additionally, in his presentation, Dr. Galsky noted significant benefits in terms of distant metastasis-free survival in both patient populations. However, Dr. Van Der Heijden noted that the benefit of adjuvant therapy appeared to be limited to those patients with bladder, rather than upper tract, urothelial cancers. Additionally, the benefit appeared to be realized most by those who had previously received neoadjuvant chemotherapy. This is particularly important as previous meta-analyses have demonstrated a benefit to adjuvant chemotherapy among patients who did not receive neoadjuvant exposure. Thus, he suggested for patients in this situation, that adjuvant chemotherapy may be more appropriate than adjuvant nivolumab, given proven benefits in rPFS (HR 0.72) and overall survival (HR 0.82).
Looking forward, he asked the question of when overall survival data will be considered in the CheckMate 274 trial. As highlighted in the study protocol, this will be an event driven determination, with approximately 404 events (57% of the study cohort of 709) required for final analysis. Thus, he postulated that this may take many years.
Dr. Van Der Heijden concluded his presentation by noting that the CheckMate 274 data shows that the DFS curves remain separated over time. As we apply this to our practices, in the PDL1-positive subset, he noted this data to be convincing, even in the absence of overall survival data. However, for those with PD-L1 negative disease and those who had not received neoadjuvant chemotherapy, he emphasized the importance of overall survival data. Additionally, he closed by noting that the results of CheckMate 274 conflict with Imvigor010 – the pending results of AMBASSADOR may serve to adjudicate and tie-break these differences.
Presented by: Michiel Simon Van Der Heijden, MD, PhD, Netherlands Cancer Institute

