(UroToday.com) On the second day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2023 focussing on urothelial cancer, the Poster Session B: Prostate Cancer and Urothelial Carcinoma included a presentation from Dr. Sanjana Ranganathan utilizing data from the National Cancer Database to compare treatment outcomes for patients with metastatic urothelial carcinoma of the bladder who received chemotherapy, immunotherapy, or a combination approach in first-line treatment.
For patients with metastatic urothelial carcinoma of the bladder (mUC), first line therapy is platinum-based chemotherapy for those who are eligible and PD1/L1 inhibitors in selected patients. To date, multiple combination chemo-immunotherapy trials have failed to show a clear benefit over chemotherapy alone. These authors sought to evaluate clinical and sociodemographic factors associated with receipt of first-line chemotherapy, immunotherapy or combination chemo-immunotherapy treatment for metastatic bladder cancer using real-world data, and to further examine differences in overall survival (OS) based on treatment approach.
To do so, they used the National Cancer Database to identify patients with stage IV UCB diagnosed between 2014 and 2018, who were treated with first-line immunotherapy, chemotherapy, or combination treatment. They used multivariable logistic regression modeling to determine factors associated with treatment receipt. To examine survival outcomes, the authors used an extension of inverse probability treatment weighting (IPTW) to balance clinical and sociodemographic differences between treatment groups prior to adjusted Kaplan-Meier survival analysis and multivariable Cox proportional hazards regression.
They identified 4,169 patients with mUC who were included, of whom 3,255 (78.1%) were treated with chemotherapy, 601 (14.4%) with immunotherapy, and 313 (7.5%) with combination treatment. Multivariable analysis identified increasing age (RRR: 1.07, 95% CI, 1.06-1.08), comorbidity burden (Charlson-Deyo 2, RRR: 1.65, 95% CI, 1.21-2.24 and Charlson-Deyo 3, RRR: 2.11; 95% CI, 1.51-2.93), and treatment at an academic facility (RRR: 1.26; 95% CI, 1.03-1.53) as independent predictors of receiving immunotherapy. Treatment at an academic facility (RRR: 1.29, 95% CI, 1.01-1.65) was associated with receipt of combination treatment.
After IPTW, combination therapy (hazard ratio [HR]: 0.72; 95% CI, 0.62-0.83), but not immunotherapy alone, was associated with improved survival compared to chemotherapy. However, this analysis is limited by an inability to determine platinum eligibility, and residual confounding inherent in observation research methodology.
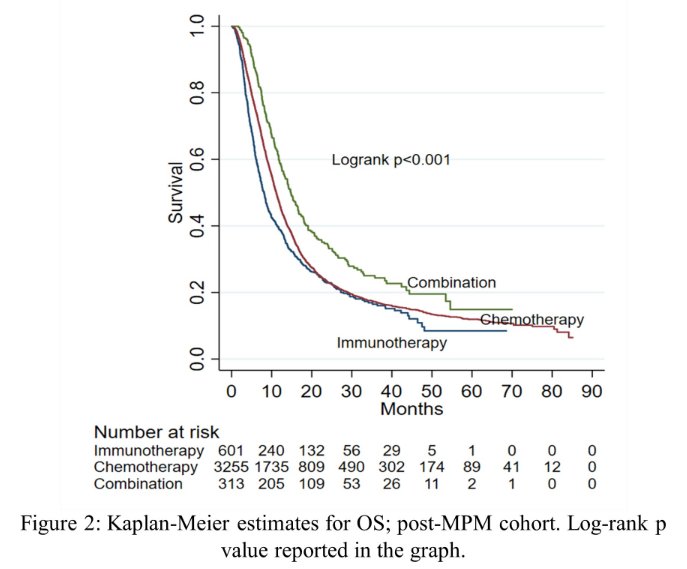
In spite of this, the authors conclude that patients with older age and more comorbidities were more likely to receive immunotherapy than chemotherapy for first-line treatment of metastatic urothelial carcinoma of the bladder. Modest real-world utilization of chemo-immunotherapy was observed to be higher in academic centers and was associated with improved survival compared to chemotherapy. However, prospective data are necessary to identify patients who may benefit from combination chemo-immunotherapy.
Presented by: Sanjana Ranganathan, MBE | Department of Urology, Houston Methodist Hospital

