(UroToday.com) The 2022 GU ASCO Annual meeting included hot topics in the urothelial carcinoma session featuring a presentation by Dr. Shaista Hafeez discussing new evidence to optimize bladder preservation. Dr. Hafeez notes that bladder preservation in muscle invasive bladder cancer relies on (i) appropriate candidate selection, (ii) developments in radiotherapy delivery, (iii) and biological personalization of radiotherapy delivery. The problem with candidate selection is that this is a heterogeneous population that may not derive benefit, as well as treatment potentially being toxic.
Diffusion-weighted MRI may be used as a biomarker for these patients, utilizing the VI-RADS scoring system. The rationale and aim of VI-RADS was to define a standardized approach to imaging and reporting mpMRI for bladder cancer, defining the risk of muscle invasion. Furthermore, VI-RADS was created through a consensus using existing literature. The now landmark VI-RADS paper was published in European Urology in 2018.1 The scoring is applicable to untreated patients and to treated patients having only received a diagnostic TURBT, but prior to re-TURBT. mpMRI is best performed before or at least 2 weeks after TURBT, bladder biopsy or intravesical treatment. Administration of an intramuscular antispasmodic agent is recommended, in addition to adequate bladder distention. MRI does not necessarily have the ability to visualize all of the histological bladder wall layers, however it is able to assess size, location, multiplicity, and morphology. A 5-point VI-RADS score is generated using the individual T2W, DWI, and DCE MRI categories and suggests the probability of muscle invasion. The dominant sequences for risk estimates are DWI (first) and DCE (second, especially if DWI is sub-optimal). The T2 sequence (structural category) is helpful as a first pass guide.
The VI-RADS 1.0 scoring is as follows:
- VI-RADS 1: SC CE and DW category 1 (muscle invasion is highly unlikely)
- VI-RADS 2: SC, CE and DW category 2; both CE and DW category 2 with SC category 3 (muscle invasion is unlikely to be present)
- VI-RADS 3: SC, CE, and DW category 3; SC category 3, CE or DW category 3, and the remaining sequence category 2 (the presence of muscle invasion is equivocal)
- VI-RADS 4: At least SC and/or DW and CE category 4; the remaining category 3 or 4 SC category 3 plus DW and/or CE category 4; SC category 5 plus DW and/or CE category 4 (muscle invasion is likely)
- VI-RADS 5: at least SC plus DW and/or CE category 5; the remaining category 4 or 5 (invasion of muscle and beyond the bladder is very likely)
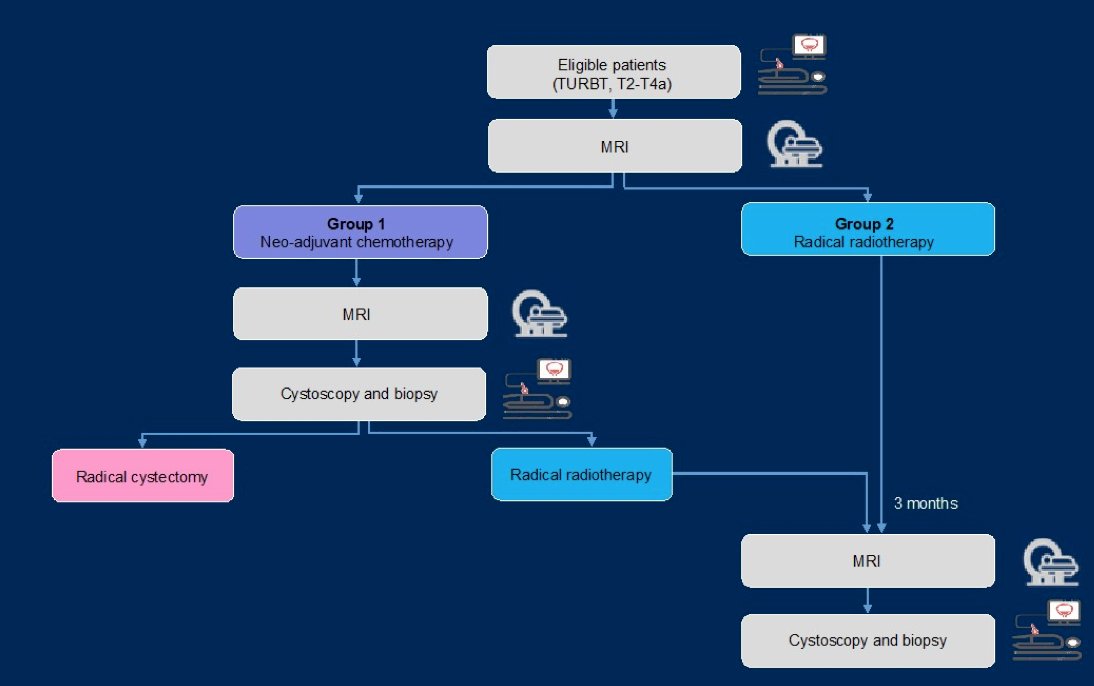
A potential algorithm for assessing treatment response incorporating mpMRI and VI-RADS is as follows:
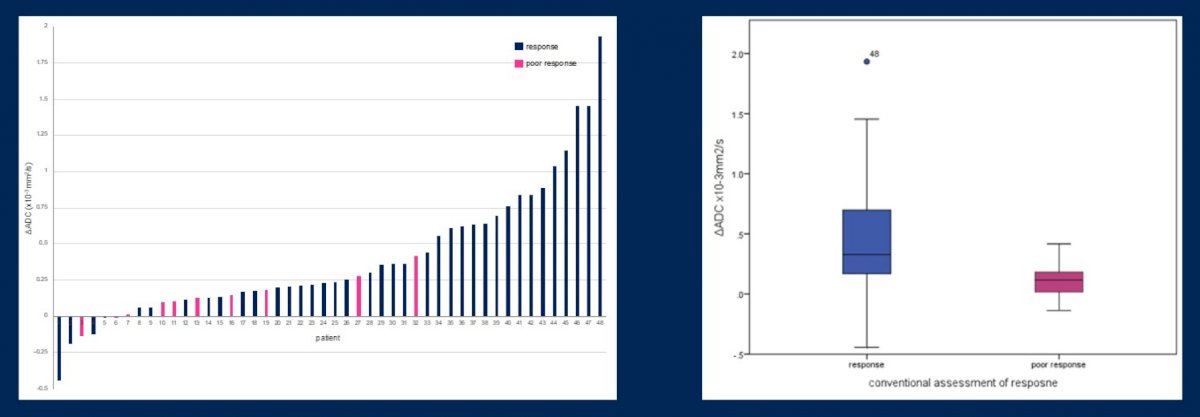
A previously reported phase I study assessed the change in ADC following neoadjuvant chemotherapy among 48 patients. A change in ADC 75th percentile predicted neoadjuvant chemotherapy response with a sensitivity of 79.0%, specificity of 90.0%, PPV of 96.8%, and NPV of 52.9%:
In this trial, after a median follow-up of 103 months (95% Ci 63.9-141.7), 31.3% patients were alive and disease free. The median overall survival was 31 months (95% CI 18.5-86.9), and time to cystectomy was 44 months (95% CI 10.0-76.2).
One of the problems in radiotherapy delivery is the changing contours of a filled vs voided bladder and the influence of rectal filling. To ameliorate these difficulties Dr. Hafeez notes the utility of image-guided adaptive radiotherapy, choosing the best fit plan based on the anatomy seen on the day of treatment and not relying exclusively on the planning CT:
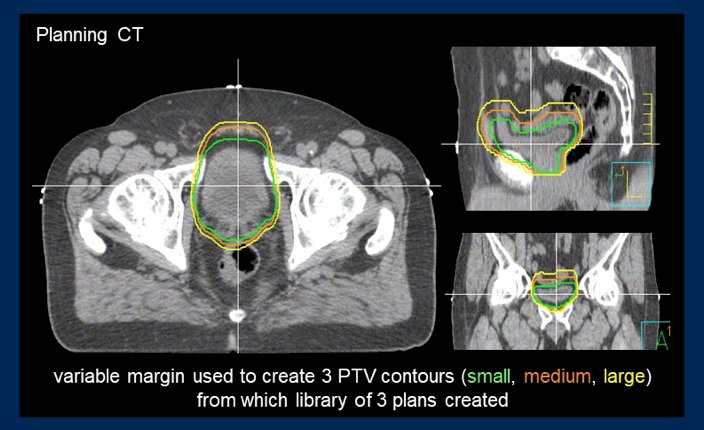

Dr. Hafeez’s group previously lead and reported results of a phase I study assessing dose escalated bladder radiotherapy. Inclusion criteria for this trial included unifocal disease T2a-4aN0M0 and planning for a radiotherapy boost to the tumor with either 68 Gy, 70 Gy, 72 Gy, or 74 Gy (dose escalation phase). The maximum tolerated dose was determined as the dose level with an estimated probability of <15% >Grade 3 late toxicity and observed rate of <50% acute grade 3 and <10% acute grade 4 toxicity. Among 59 patients including in this study, the median age was 74 years (range: 50-90), the majority were male (85%), the most common cT stage was cT2 (71%), and 61% of patients receiving neoadjuvant chemotherapy. The study schema for this trial is as follows:

The maximum tolerated dose in this study was 70 Gy, with an 18% grade 3 genitourinary acute toxicity rate, and 8% grade 3 gastrointestinal acute toxicity rate. The late RTOG grade 3 toxicity rate was 13% and grade 4 toxicity rate was 2%. With regards to bladder preservation, the cystectomy rate was 6% as four patients required salvage cystectomy.
Dr. Hafeez then highlighted the ongoing RAIDER trial, a randomized phase II trial of adaptive image guided standard or dose escalated tumor boost radiotherapy in treatment of transitional cell carcinoma of the bladder (NCT02447549). This trial will randomize patients 1:1:2 to standard planning and delivery radiotherapy vs adaptive image guided tumor focused radiotherapy vs adaptive image guided dose escalated tumor boost radiotherapy:

With regards to the biological personalization of radiotherapy, Dr. Hafeez notes that radiotherapy resistant areas of the tumor may be identifiable. Their group presented work assessing normal tissue sparing with diffusion weighted MRI informed tumor boost among 21 patients undergoing bladder radiotherapy [2]. For treatment, the tumor boost volume (GTV) was delineated on the radiotherapy planning CT (GTVCT). All tumors seen on T2 image were identified on DWI, with DWI acquisition associated with ~45% target volume reduction (p=0.002) and a >60% reduction to high dose bowel and normal bladder constraints:

Dr. Hafeez concluded her presentation discussing new evidence to optimize bladder preservation with the following summary statements:
- Quantitative DWI predicts treatment outcome and provides prognostic information, although prospective multi-center validation is necessary
- Dose escalation to 70 Gy in 32 fractions to the bladder tumor is feasible with acceptable toxicity
- RAIDER (NCT02447549) is an ongoing multi-center phase II randomized control trial of adaptive dose escalated bladder radiotherapy
- DWI acquisition during treatment may provide opportunities for radiotherapy adaption
Presented By: Shaista Hafeez, BSc (hons) MSc MBBS MRCP FRCR PhD, Clinical Oncologist, The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, London, UK
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022
References:
- Panebianco V, Narumi Y, Altun E, et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting and Data System). Eur Urol 2018 Sep;74(3):294-306.
- Chan K, Warren-Oseni K, Abdel-Aty H, et al. Normal tissue sparing with diffusion weighted MRI informed tumour boost in bladder radiotherapy. Radiotherapy Oncology. 2019;133:S455-S456.


