(UroToday.com) The 2022 GU ASCO Annual meeting included a potpourri of hot topics in urothelial carcinoma session featuring a presentation by Dr. Surena Matin discussing endoscopic management of low-grade upper tract urothelial carcinoma. Dr. Matin notes that there are several challenges to the management of low-grade upper tract urothelial carcinoma, which include (i) the risk of upgrading, (ii) impact of disease volume (low volume disease – nephroureterectomy is overtreatment; high volume/multifocal disease – poor kidney preserving options), (iii) the number of endoscopic procedures/anesthetics in an elderly population, (iv) stent symptoms and ureteral strictures, and (v) minimal high-level data.
There are several established therapies for the treatment of low-grade upper tract urothelial carcinoma. First, the most established therapy is nephroureterectomy. The pros of a nephroureterectomy are that it is a single effective treatment, eliminates the risk of ipsilateral recurrence, and is likely optimal in elective settings for high volume or multifocal disease. However, a nephroureterectomy comes at the highest cost of GFR decline in most cases.
Second, a distal ureterectomy is also an established therapy for low grade disease, with the pros being a single effective treatment that maintains kidney function. However, there are several cons associated with a distal ureterectomy, including a risk for ipsilateral recurrence and that it is reserved for highly selective cases: unifocal disease in the distal ureter, no active bladder disease, with an array of available reconstruction options (ie. psoas hitch, Boari flap, ileal ureter).
Third, ureteroscopy is an established therapy for low grade upper tract urothelial carcinoma. The pros of ureteroscopy are that it is minimally invasive, repeatable, is associated with high cancer specific survival, and maintains kidney function. The cons of ureteroscopy are that it is more invasive than cystoscopy and associated with more symptoms (ie. stent irritation), there is a stricture risk, there may be multiple procedure, it is suboptimal for high volume and multifocal disease, likely associated with high cost when >1 procedure is needed, and there are no great options for preventing recurrences.
Dr. Matin notes that endoscopy + adjuvant topical therapies for upper tract urothelial carcinoma result in cancer specific survival rates of 70-100%, renal preservation rates of 71% and recurrence rates of 12-90%. Topical agents have included a variety of agents, including mitomycin C, BCG, gemcitabine and thiotepa. A variety of delivery methods have been used, including antegrade instillation via nephrostomy tube and retrograde instillation via a ureteral catheter; refluxing via a ureteral stent is not considered a reliable to be effective.
Dr. Matin then provided an overview of ureteroscopy for the uninitiated:
Tools –
- Use of a 7-9F, 115cm long scope which allows a single <3Fr instrument (biopsy forcep, basket, laser fiber, bugbee electrode, etc)
- Constant saline irrigation under pressure
- Few tools are specifically made for tumors, so being innovative is important
Concepts –
- We are unable to biopsy more than just superficial urothelium, thus muscle in the specimen is not a realistic (or safe) expectation
- For a biopsy, the entire instrument has to be removed and reinserted with each sampling, which is a benefit of a placing a ureteral access sheath
- There is limited progress with each intervention
- A stent is frequently indicated when biopsies are done
Dr. Matin emphasizes that European Association of Urology low-risk criteria patients are the most ideal setting for ureteroscopy. The following figure highlights the criteria for these low-risk patients:1
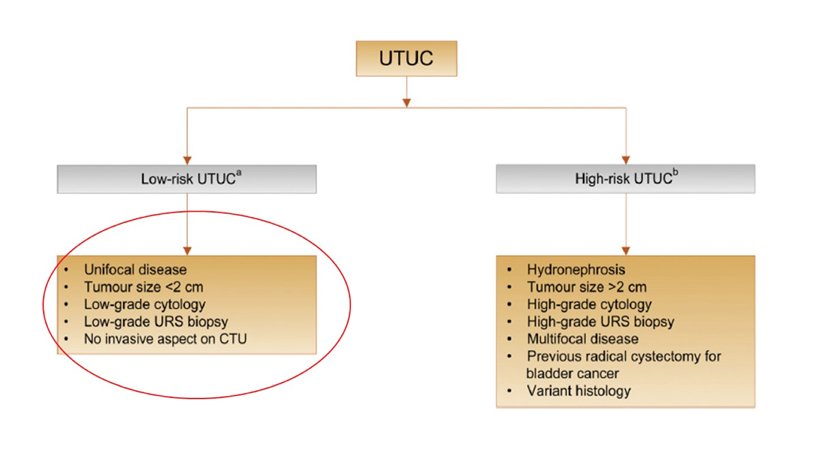
In Dr. Matin’s opinion, this represents “very low-risk” disease, both low risk for disease progression and for successful ureteroscopic management. Importantly, this does not predict recurrence potential.
Mitomycin-C was discovered in 1963 and has been used for decades. In 2016, mitomycin C was tested in combination with a novel reverse polymer hydrogel, which is thick liquid at cold temperatures, gel at body temperature, and dissolves in liquid (ie. urine). This was engineered as a sustained release of mitomycin C-hydrogel formulation.
The OLYMPUS trial was a phase 3, open-label, single arm trial designed to assess the efficacy, safety, and tolerability of UGN-101 in patients with low grade, noninvasive upper tract urothelial cancer.2 Eligible patients had either primary or recurrent biopsy-proven low-grade upper tract urothelial carcinoma of the renal pelvis or calyces, diagnosed in the two months prior to trial screening. Enrolled patients received six once-weekly instillations of UGN-101 as an induction course, administered via retrograde instillation with ureteral catheterization. Four to six weeks following initial treatment, patients received their primary disease evaluation including ureteroscopy, selective upper tract cytology, and for-cause biopsy where indicated. Complete response was defined as a negative endoscopic evaluation and the absence histologic or cytologic evidence of disease. Patients who experienced a complete response were then offered ongoing monthly maintenance is offered for 11 instillations or until first recurrence.
Among 110 patients screening, 74 were enrolled and 71 patients received treatment of which 42 patients (59%, 95% CI 47-71%) had a complete response at the time of primary disease evaluation. Of the remainder, 8 (11%) had a partial response, 12 (17%) had no response, 6 (8%) had newly diagnosed high-grade disease, and 3 (4%) had an indeterminate response. Twelve-month durability could be assessed in 20 patients. Of these 20 patients, 14 (70%) showed ongoing durability of their complete response and 6 had a documented recurrence during follow-up. However, none of these patients progressed to high-grade or invasive disease. Among those with complete response at primary disease evaluation, 84% (95% CI 71-97%) remained disease free at 12 months. Median time to recurrence was reported as 13 months (95% CI 13 months to not estimable):
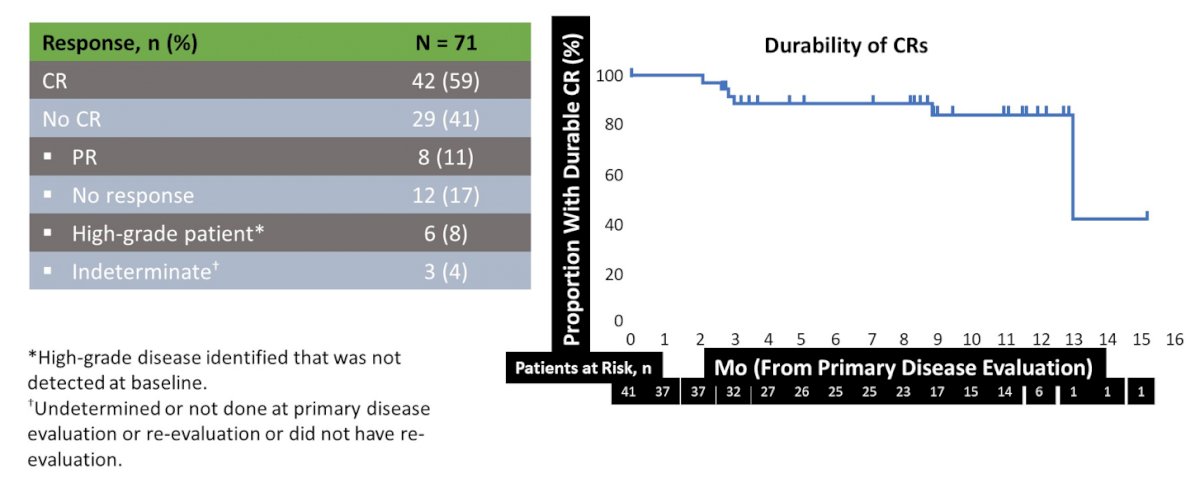
The FDA approval of Jelmyto in April 2020 for primary chemoablation of low-grade upper tract urothelial carcinoma based on results from the OLYMPUS phase III study, which establishes a new standard of care as chemoablation after diagnostic ureteroscopy. The pros of mitomycin gel are that it is minimally invasive, offers chemoablation of low-volume residual tumors, 59% of patients had a complete response with 80% durability at 1 year, it likely prevents recurrences, and it maintains kidney function. The cons of mitomycin gel are that there is an associated stricture risk of 30-40%, it is expensive, it needs retrograde or antegrade access and it is suboptimal for high volume disease.
Dr. Matin noted that there are several practical concepts that are important to highlight for the utilization of Jelmyto, specifically with regards to the ureteral stricture rate seen in the trial and in clinical practice. Steroids (Medrol dose pack) and treatment holiday should be utilized at the first suggestion of any ureteral narrowing, usually seen by the 3rd of 4th treatment. Although untested (but practiced by Dr. Matin and others), is that for complete laser ablation, clinicians should give retrograde treatment as adjuvant treatment of 2-3 doses, and that for incomplete laser ablation, all six doses should be given by nephrostomy tube.
Several important clinical trials are going in this disease space. A phase 1b trial of infigratinib before ureteroscopy or nephroureterectomy (n=20) has enrolled 12 patients since May 2021, and 9 have completed treatment. Thus far, the tolerability is 78% and there is plans to extend to a phase 2 platform of 50 patients. Potential advantages of this approach are that infigratinib is an oral compound and treatment may be utilized irrespective of size or multifocality (perhaps grade?). Dr. Matin also highlighted the phase 3 ENLIGHTED trial of safety and efficacy of padeliporfin vascular PDT for low-grade upper tract urothelial carcinoma.
Dr. Matin concluded with several key take-away messages from his presentation discussing the endoscopic management of low-grade upper tract urothelial carcinoma:
- For low-volume, low-grade upper tract urothelial carcinoma, consider maximal ureteroscopic laser ablation followed by mitomycin gel therapy
- For high-volume, low-grade upper tract urothelial carcinoma, with a healthy contralateral kidney with anticipated good post-nephroureterectomy GFR, a nephroureterectomy is still reasonable
- Distal ureterectomy with ureteroneocystostomy remains a good kidney-preserving option for unifocal high volume or circumferential distal ureter tumors in highly selected patients
- Targeted (anti-FGFR) therapy appears promising and being explored as treatment in a clinical trial
- Photodynamic therapy is being explored in a phase 3 clinical trial
Presented By: Surena F. Matin, MD, University of Texas MD Anderson Cancer Center, Houston, TX
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022
- Roupret M, Babjuk M, Capoun O, et al. European Association of Urology Guidelines on Upper Tract Urothelial Carcinoma: 2020 Update. Eur Urol 2020 Jun 24:S0302-2838(20)30427-9.
- Kleinmann N, Matin SF, Pierorazio PM, et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): An open-label, single-arm, phase 3 trial. Lancet Oncol 2020 Jun;21(6):776-785.


