(UroToday.com) The 2022 GU ASCO Annual meeting included a prostate cancer session highlighting work from Dr. Morgan Roupret and colleagues presenting preliminary results of the "DUO" observational study, a quality of life assessment and cross-sectional follow-up of prostate cancer patients treated with degarelix. The use of androgen deprivation therapy (ADT), has increased over time in the treatment of prostate cancer, however ADT is accompanied by side effects, some of which may increase the cardiovascular risk. The aim of this study was to evaluate the prevalence of cardiovascular risks and quality of life at the initiation of degarelix, a GnRH antagonist, and 6 months later, in patients with advanced hormone-sensitive prostate cancer.
The DUO study is a PASS, pharmaco-epidemiologic, longitudinal, multicenter observational study. It prospectively enrolled prostate cancer patients during the first 6 months of treatment with degarelix. The study was carried out in 46 French urology centers. At Day 0 and Month 6, the prevalence of cardiovascular, osteoporotic, metabolic, mood disorder, geriatric and sexual morbidity and risk factors were recorded using validated tools and questionnaires, and the EQ-5D questionnaire was used to measure quality of life. The evolution of the disease (PSA levels) and the tolerance of the treatments were also reported.
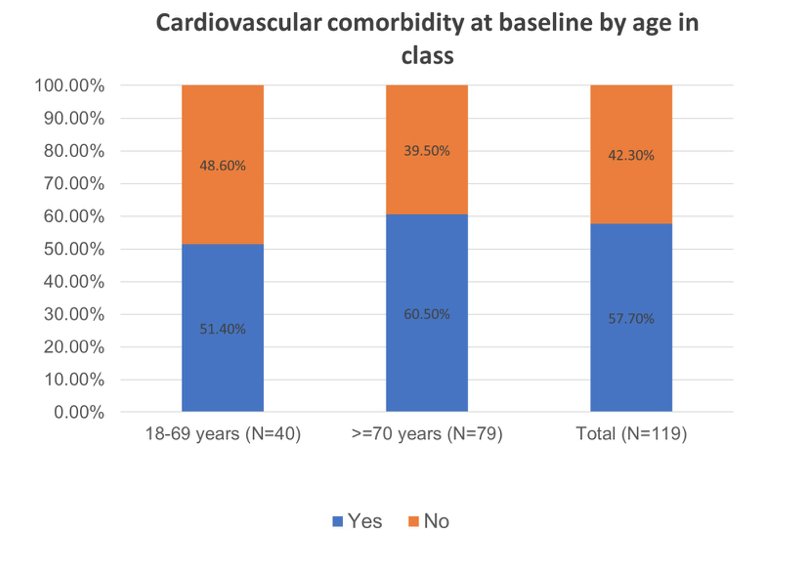
There was a total of 124 patients with advanced hormone-sensitive prostate cancer (of whom 26.6% were metastatic) included. At Day 0, 57.7% of patients had a cardiovascular morbidity and cardiovascular risk factors:
Metabolic co-morbidities were also present in 60.6% of patients, osteoporotic risk factors in 34%, sexual disorders in 58%, and mood disorders in 36%:
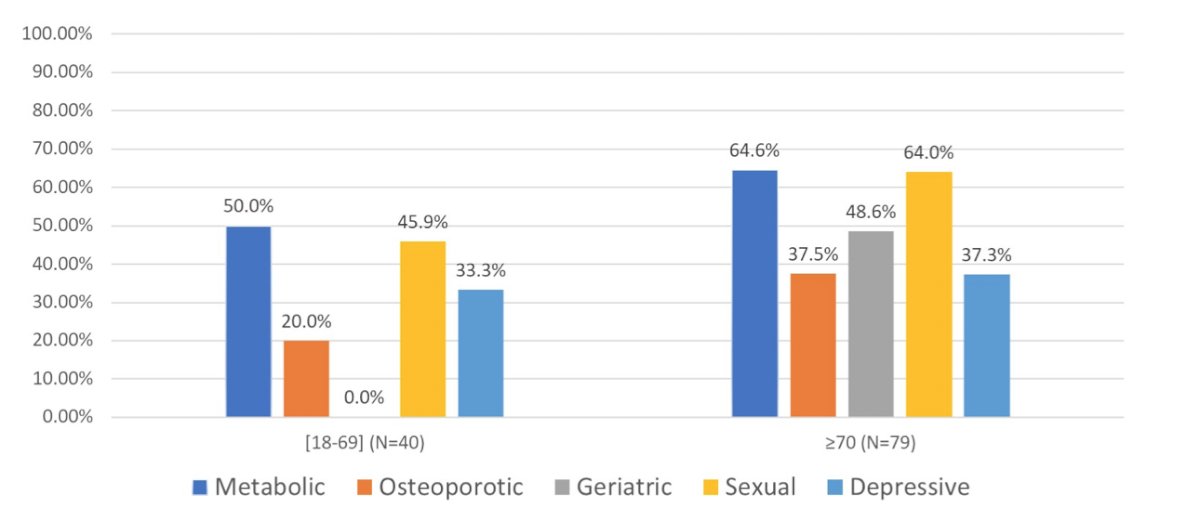
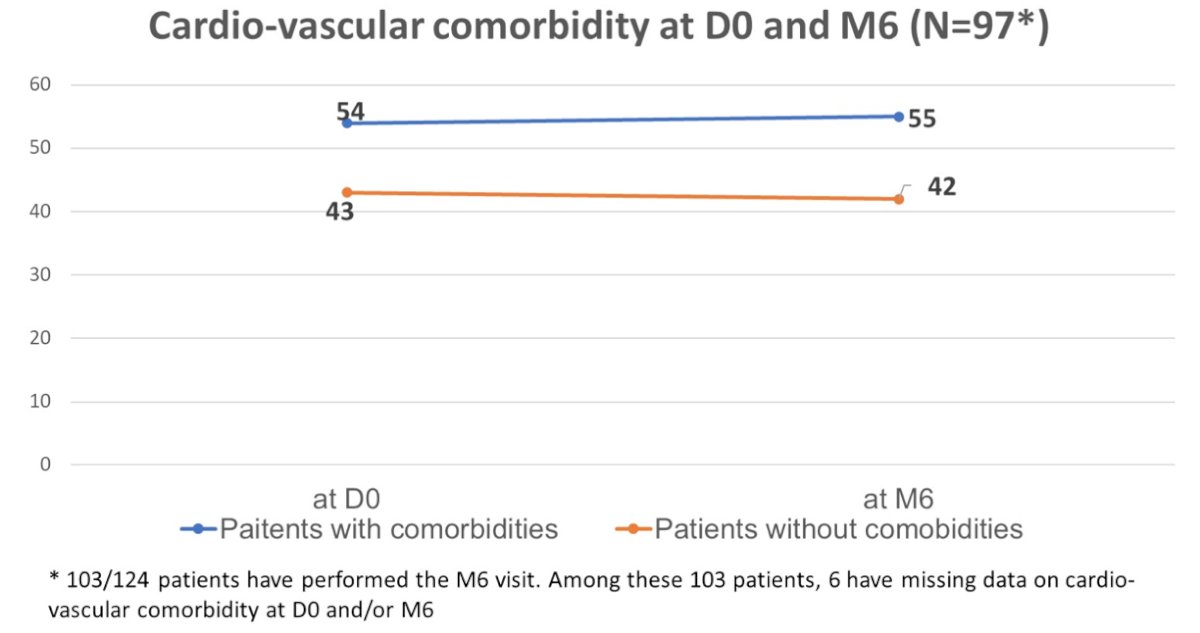
ONCO-G8 score ≤ 14 was observed in 45.6% of patients aged ≥ 70 years. Over time, the percentage of patients with cardiovascular co-morbidities remained stable (56.7%), and the rates of osteoporotic, metabolic, sexual, and mood-related morbidity and risk factors also remained unaltered:
Likewise, quality of life remained stable between Day 0 and Month 6 (Scores: 0.765 and 0.813, respectively). At the same time, the median PSA rate decreased by -98.4% from Day 0 (p < 0.001). Overall, there were 42 adverse events were reported in 22 patients, and degarelix-related adverse events were reported in 4.4% of patients.
Dr. Roupret concluded his presentation of the preliminary results of the "DUO" observational study with the following summary statements:
- The results of the DUO study showed high prevalence of cardiovascular co-morbidity and risk factors (57.7%) in patients with advanced prostate cancer
- There was no significant increase in cardiovascular co-morbidities and risk-factors during the first 6 months of treatment with degarelix as it was observed for osteoporotic, metabolic, sexual, and mood-related disorders
- This GnRH antagonist was well tolerated without major impact on quality of life
- Assessment of multiple co-morbidities at the initiation and during ADT is mandatory for the optimal management of patients.
Presented by: Morgan Roupret, Hospital Surgeon, Hôpital Pitié-Salpétrière, AP-HP, Institut Mutualiste Montsouris, University Paris-Descartes, Paris, France
Co-Authors: Francois Rozet, Diane Dalila Delattre, Hugo Lacour, Fallouh Ghassan, Matthieu Durand, Pierre Mongiat Artus
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022


