(UroToday.com) The 2022 GU ASCO Annual meeting included a prostate cancer session featuring work from Dr. Andrei Gafita and colleagues presenting results of RECIP, an international multicenter study with a novel framework for evaluating treatment response using PSMA-PET/CT in patients with metastatic castration resistant prostate cancer (mCRPC). PSMA-PET/CT has previously been shown to have superior diagnostic accuracy compared to conventional imaging, but there is little evidence for its prognostic role in treatment monitoring. This study aimed to develop a novel framework for Response Evaluation Criteria In PSMA-PET/CT (RECIP) 1.0 and a composite response classification which combines responses by PSA measurements and by RECIP 1.0 (PSA+RECIP).
This was an international, multicenter, retrospective study of 124 men with mCRPC who underwent 177Lu-PSMA therapy and received PSMA-PET/CT at baseline and at interim at 12 weeks. Pairs of PSMA-PET/CT at baseline and interim PSMA-PET/CT were interpreted by consensus among three blinded readers for appearance of new lesions. The study design is as follows:
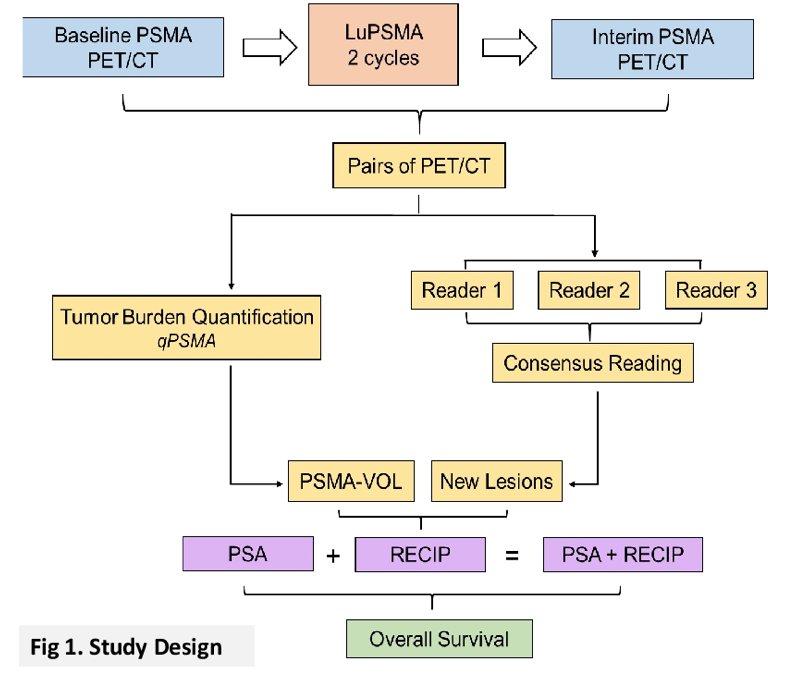
Tumor lesions were segmented and total PSMA-positive tumor volume was obtained. Appearance of new lesions and changes in PSMA-positive tumor volume were combined to develop RECIP 1.0, which was defined as:
- RECIP-Complete response: absence of any PSMA-ligand uptake on interim PSMA-PET/CT
- RECIP-Partial response: decline ≥30% in PSMA-positive tumor volume and no appearance of new lesions
- RECIP-Progressive disease: increase ≥20% in PSMA-positive tumor volume and appearance of new lesions
- RECIP-Stable disease: any condition but RECIP-partial response or RECIP-progressive disease
Changes in PSA levels at 12 weeks by PCWG3 were recorded. Responses by PSA+RECIP were defined as: response (PSA decline ≥50% or RECIP-partial response/complete response) and progression (PSA increase ≥25% or RECIP-progressive disease). This study's primary outcome measure was the prognostic value of RECIP 1.0 for overall survival (OS). Secondary outcome measure was the prognostic accuracy (C-index) of PSA+RECIP versus PSA responses.
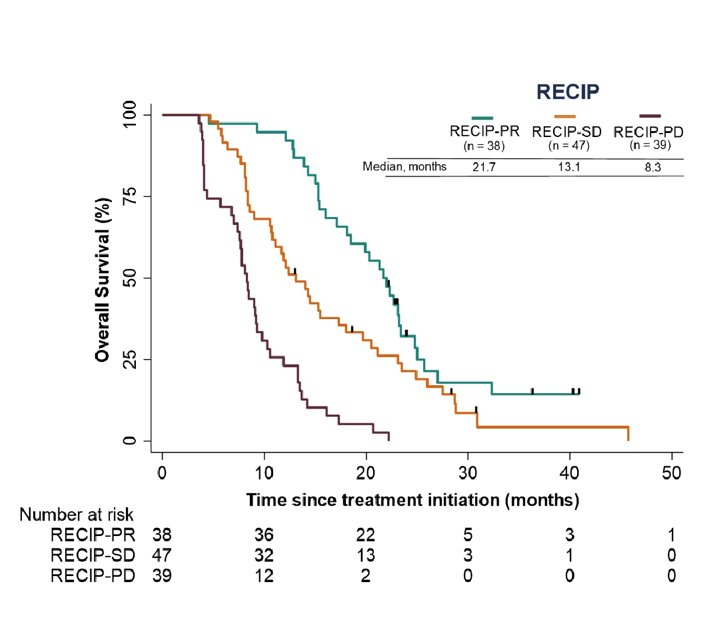
Patients with progressive disease (RECIP-progressive disease; n=39; 8.3 months) had shorter OS compared to patients with stable disease (RECIP-stable disease; n=47; 13.1 months; p<0.001) and to those with partial response (RECIP-partial response; n=38; 21.7 months; p<0.001):
PSA+RECIP had superior C-indices in identifying responders and progressors compared to PSA only: 0.66 versus 0.63 (p=0.028) and 0.65 versus 0.62 (p=0.044), respectively.
Dr. Gafita concluded this presentation of RECIP with the following take-home messages:
- PSMA-PET/CT by RECIP 1.0 is prognostic for OS and can be used as a response biomarker to monitor efficacy of 177Lu-PSMA in men with mCRPC
- PSA+RECIP may be used as a novel composite endpoint in mCRPC clinical trial design
- Validation of the findings in a prospective setting is warranted
Presented by: Andrei Gafita, University of California, Los Angeles, Los Angeles, CA
Co-Authors: Isabel Rauscher, Manuel Weber, Boris A. Hadaschik, Hui Wang, Wesley R Armstrong, Robert Tauber, Tristan Grogan, Johannes Czernin, Matthew Rettig, Ken Herrmann, Jeremie Calais, Wolfgang A Weber, Matthias R. Benz, Wolfgang Peter Fendler, Matthias Eiber
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022


