(UroToday.com) The 2022 GU ASCO Annual meeting included a session on the optimization management of localized prostate cancer, specifically looking at artificial intelligence (AI), active surveillance, and intervention, featuring a presentation by Dr. Stacy Loeb discussing active surveillance for patients with intermediate risk disease. Dr. Loeb notes that there has been an increase in the utilization of active surveillance in the United States for patients with intermediate risk disease over the last several years, notable for an increase in active surveillance/watchful waiting from 5.8% in 2010 to 9.6% in 2015. However, this is still substantially lower than the utilization of active surveillance in other countries. In a study looking at data from Sweden, Dr. Loeb and colleagues noted that in 2014 the active surveillance uptake was 91% for very low risk disease, 74% for low-risk, and 19% for intermediate risk prostate cancer:1
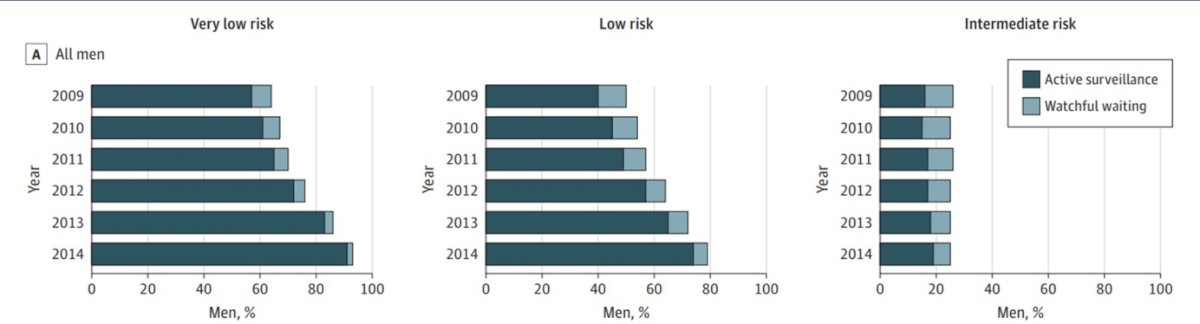
Dr. Loeb notes that there is greater uptake of active surveillance for intermediate-risk prostate cancer based on PSA only versus Grade Group 2 disease (2009-2014):

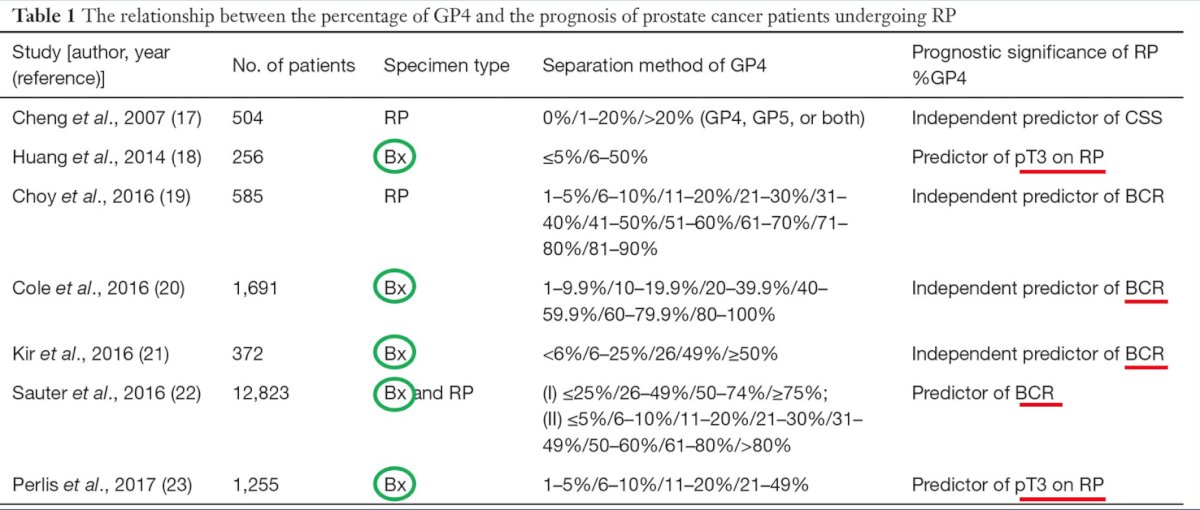
However, defining men with intermediate risk disease suitable for active surveillance has been somewhat challenging. Dr. Loeb notes that there is no difference in upgrading or adverse pathology between men with GG1 and PSA 10-15 ng/ml compared to low-risk disease. But, men at intermediate-risk due to PSA > 15 ng/mL or GG2 have higher risk of adverse pathology. Several series have assessed the importance of percent pattern 4, which has been shown to be a predictor of pT3 on radical prostatectomy and with biochemical recurrent disease:
Other additional considerations on biopsy that should be taken into consideration are the presence of cribriform histology and intraductal carcinoma. The EAU guidelines strongly recommend these patients be excluded from active surveillance protocols. Dr. Loeb notes that the NCCN guidelines highlight many scenarios in which germline testing should be considered for men with prostate cancer, but specific to non-high risk (ie. those that may be considered for active surveillance), this includes patients with Ashkenazi Jewish ancestry and men with a family history of high-risk germline mutation. Germline testing has historically been poor among localized prostate cancer with only 28% of urologists recommending/performing testing in men with intermediate risk disease, Ashkenazi Jewish, with no family history of prostate cancer.
Carter et al.2 previously assessed whether germline mutations are associated with grade reclassification in patients undergoing active surveillance. Among 1,211 men on surveillance, 289 experienced grade reclassification, 11 of 26 with mutations in a three-gene panel, and 278 of 1185 non-carriers (aHR 1.96, 95% CI 1.004-3.84, p=0.04). Reclassification occurred in six of 11 carriers of BRCA2 mutations and 283 of 1200 non-carriers. Importantly, men with BRCA/ATM pathogenic variants had a higher risk of reclassification during active surveillance compared to non-mutation carriers:

Dr. Loeb then discussed monitoring protocols, which based on the most recent version of the NCCN guidelines (v3.2022) should be considered as follows:

Dr. Loeb emphasized that risk calculators can also be helpful in these situations when counseling patients. One example is the Canary Pass Risk calculator that imputes age, BMI, PSA, prostate volume, time since diagnosis of prostate cancer, number of cores on the most recent biopsy, and the total number of cores, subsequently outputting the risk of Gleason upgrade at the time of next biopsy. Work from Dr. Loeb and her colleagues from Sweden showed that for intermediate risk disease, 41% discontinued active surveillance at 5 years, compared to 35% with very low risk and 33% with low risk disease.3 Additionally, Gleason 7 versus Gleason 6 disease was not significantly associated with discontinuation of active surveillance. In the Canary PASS cohort, which included 154 with GG2 prostate cancer, there was no difference in reclassification for GG1 versus GG2, however, GG2 was associated with more treatment without reclassification (similar pathologic disease).
Dr. Loeb then discussed the importance of lifestyle promotion during active surveillance. The American Cancer Society Prostate Cancer Survivorship Care guidelines suggest that we should be counseling our patients to:
- Maintain a healthy weight
- Engage in at least 150 minutes/week of physical activity
- Eat a diet high in fruit, vegetables, and whole grains
Additionally, we should be assessing our patients for tobacco use and refer them appropriately for tobacco cessation counseling.
In a study from 2005, Ornish et al.4 evaluated the effects of comprehensive lifestyle changes on PSA, treatment trends and serum stimulated LNCaP cell growth in men with early, biopsy proven prostate cancer after one year. Men in the intensive lifestyle program intervention arm (vegan diet with supplements, moderate aerobic exercise, stress management) compared to a control group (without lifestyle intervention) had a lower rate of progressing to treatment, a 4% decrease in PSA, a decrease in cholesterol levels.
The ERASE randomized control trial was recently published in JAMA Oncology,5 which was undertaken to examine the effects of exercise on cardiorespiratory fitness and biochemical progression in men with prostate cancer who were undergoing active surveillance. There were 52 men randomized to high-intensity interval training 3 times/week versus usual care, with the intervention arm demonstrating improved cardiopulmonary fitness and PSA reduction. These are certainly hypothesis generating results and larger trials are warranted to determine whether such improvement translates to better longer-term clinical outcomes in this setting.
Dr. Loeb finished her presentation by highlighting several future directions, including the Prostate Cancer Active Surveillance Project (PCASP – PI: Dr. Matt Cooperberg), which is the largest examination of utilization and quality of active surveillance for intermediate-risk disease. Second, Movember in partnership with Dr. Caroline Moore is leading an international effort to explore the broad aspects of active surveillance to identify the top funding priorities in active surveillance for the next 5 years and best practices for clinical care. This project involves 40+ participants, representing 10 disciplines from Australia, the UK, Europe, Canada, and the US with findings expected in April/May 2022.
Dr. Loeb concluded her presentation of active surveillance for patients with intermediate risk disease with the following summary statements:
- There is increasing use of active surveillance in intermediate-risk prostate cancer, which may be an important option to reduce downstream harms
- However, not all intermediate risk disease is the same and we must quantify the amount of pattern 4 disease and germline test when indicated
- There should be a risk-adapted strategy to follow-up testing, given that the quality of surveillance is important since there is a higher risk of metastasis
- Lifestyle modifications remain a central component for all patients on active surveillance
Presented by: Stacy Loeb, MD, NYU Langone Health, New York, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday, Feb 17 – Saturday, Feb 19, 2022
References:
- Loeb S, Folkvaljon Y, Curnyn C, et al. Uptake of active surveillance for very-low-risk prostate cancer in Sweden. JAMA Oncol 2017;3(10):1393-1398.
- Carter HB, Helfand B, Mamawala M, et al. Germline mutations in ATM and BRCA1/2 are associated with grade reclassification in men on active surveillance for prostate cancer. Eur Urol. 2019 May;75(5):743-749.
- Loeb S, Folkvalijon Y, Makarov DV, et al. Five-year Nationwide follow-up study of active surveillance for prostate cancer. Eur Urol. 2015;67:233-238.
- Ornish D, Weidner G, Fair WR, et al. Intensive lifestyle changes may affect the progression of prostate cancer. J Urol. 2005;174(3):1065-1069.
- Kang DW, Fairey AS, Boule NG, et al. Effects of exercise on cardiorespiratory fitness and biochemical progression in men with localized prostate cancer under active surveillance. The ERASE Randomized Clinical Trail. JAMA Onc. 2021 Oct 1;7(10):1487-1495.


