(UroToday.com) The 2022 GU ASCO Annual meeting included a session on the management of rare variants in genitourinary cancers and a presentation by Dr. Eric Small discussing the role of systemic therapy for the management of rare prostate cancer variants. Dr. Small started by highlighting the variant histology criteria for inclusion in this presentation:
- Real: variant is reasonably broadly accepted as existing
- Systemic: likely to identified in the context of metastatic prostate cancer, often with mCRPC
- Rare: found in 25% or less of mCRPC
- Actionable: there is some therapy available to manage the variant
- Active: phase 2 or later data with strong evidence of clinical activity
- Current: in clinical use today, and if not yet approved, with reasonable probability of informative data soon
Genotyping for somatic and structural alterations in mCRPC suggests that 80% have alterations in the androgen receptor and 57% have mutations in TP53. With regards to DNA damage homologous recombination repair (DDR) alterations, somatic mutations note BRCA2 mutations in 11%, BRCA1 in 1%, ATM in 6%, and CDK12 in 3%. Additionally, 11.8% of men with metastatic prostate cancer have germline DDR defects.1
The PROfound study recruited men with mCRPC who had progressed on previous abiraterone acetate or enzalutamide administered at the time of non-metastatic castrate-resistant prostate cancer or at the time of metastatic castrate-sensitive prostate cancer.2 Alterations were identified in one of 15 pre-specified genes involved in homologous recombination repair (BRCA 1/2, ATM, BRIP1, BARD1, CDK12, CHEK 1/2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, RAD54L). Cohort A had alterations in BRCA1, BRCA2, or ATM while Cohort B had alterations in any of the other 15 included genes. In both cohorts, patients were randomized 2:1 to olaparib vs. abiraterone or enzalutamide. The primary analysis was based on imaging-based progression free survival among patients in Cohort A. In an assessment of the primary outcome, there was a significantly improved progression-free survival in patients with mutations of BRCA1, BRCA2, or ATM (HR 0.34, 95% CI 0.25 to 0.47). Similar results were seen in the combined cohort (HR 0.49, 95% CI 0.38 to 0.63). Based on the results of the PROfound trial, the FDA granted approval for olaparib for deleterious or suspected deleterious germline or somatic homologous recombination repair (HRR) gene-mutated mCRPC that progressed with abiraterone or enzalutamide. However, Dr. Small notes that patients with ATM mutations did not have the same benefit as other alterations (HR for rPFS 1.04, 95% CI 0.61-1.87).
The TRITON2 trial assessed rucaparib 600 mg BID in patients with mCRPC associated homologous recombination repair gene alterations.3 For the patients with BRCA1/2 alteration, there was a 44% confirmed overall response rate and 51% confirmed PSA response rate while patients harboring an ATM and CDK12 alteration did not receive significant benefit. The median rPFS among 115 BRCA carriers was 9.0 months (95% CI 8.3-13.5). Based on this data, the FDA granted accelerated approval to rucaparib for men with BRCA-mutated metastatic CRPC (germline or somatic) who had prior treatment with androgen-receptor directed therapy and prior taxane-based chemotherapy.
Dr. Small notes that when considering PARP inhibitor therapy for prostate cancer, this should only be considered in patients with BRCA1/2 mutations, possibly for CDK12 mutations and not for ATM mutations. Currently, in the clinical setting, PARP inhibitors should be used in the second or third line or mCRPC following an ASI. However, after GU ASCO 2022, based on the PROpel (olaparib) and MAGNITUDE (niraparib) trials, perhaps practices will change to include these PARP inhibitors in combination with abiraterone in the treatment of first-line mCRPC. Studies are ongoing as front therapy for mHSPC given with ADT and abiraterone.
Platinum chemotherapy for DDR deficiencies has similar activity to the same mCRPC patients who respond to PARP inhibitors, with the caveat that evaluations to date have only been based on PSA50 data, with no direct comparison of platinum chemotherapy and PARP inhibitors. There are currently many unanswered questions comparing these two treatments, including efficacy, durability, resistance, quality of life, and cost.
With regards to CDK12 variants and therapeutic implications, upfront ASI works best for these patients with PSA50 responses ranging from 41%-68%, whereas PARP inhibitor utility is modest at best with PSA50 response rates <10%. Dr. Small notes that there is reason to consider checkpoint inhibitors in patients with CDK12 variants, given their genomic instability, focal tandem duplications, and increase in fusion neoantigens.
MSI-high (MLH1, MSH2) variants are present in 4% of advanced prostate cancer tumors, with pembrolizumab receiving FDA approval in 2017 in patients with MSI-high and/or d-MMR cancers. Dr. Small notes that checkpoint blockade is active in d-MMR/MSI-high prostate cancer patients, as summarized in the following table:
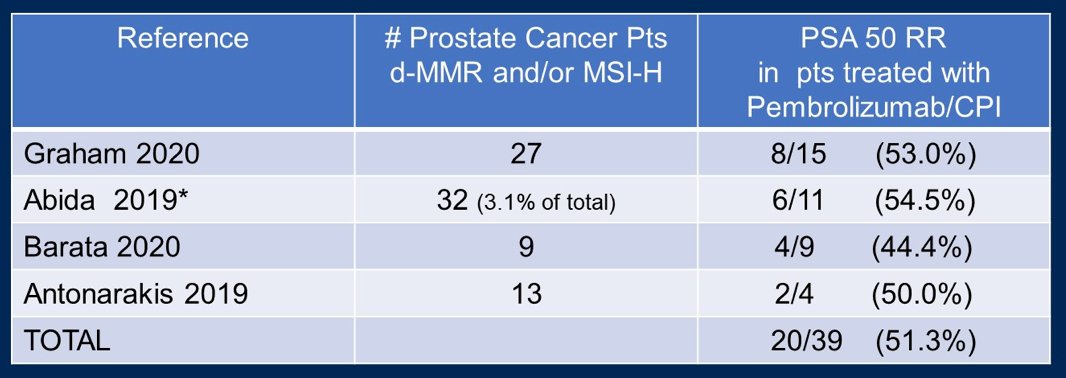
Checkpoint inhibitors should be used in prostate cancer when there are d-MMR and MSI-high mutations and possibly for CDK12 mutations. This treatment option is primarily reserved following ASI, with no evidence to support utilization instead of frontline abiraterone or enzalutamide. Of note, combination studies (checkpoint inhibitor + ASI) are pending.
Cell cycle regulation (RB1, CD4,6, cyclin D) alterations in prostate cancer do not currently have targetable treatment options. Palbociclib + ADT in RB intact mHSPC was a negative study, however, ribociclib in a single arm, phase 2 trial in combination with docetaxel in mCRPC met is rPFS endpoint.
Primary small cell prostate cancer is very rare (1% of localized prostate cancer specimens), is frequently present with metastatic disease, is commonly associated with visceral metastases, and is usually not responsive to AR-targeted treatment. There are limited series assessing treatment, most using platinum based chemotherapy regimens. Unfortunately, this variant has a dismal prognosis with poor survival. Treatment associated small cell/neuroendocrine prostate cancer has a prevalence of ~17% in patients undergoing mCRPC metastasis biopsies. Unlike primary small cell cancer, there is some AR signaling (+ AR staining), with PSA production ranging from 0.4-1500; it does not have increased risk of visceral metastasis. However, like primary small cell prostate cancer, treatment associated small cell/neuroendocrine prostate cancer is associated with poor survival. As follows is a table summarizing therapy for treatment associated small cell/neuroendocrine prostate cancer:

Dr. Small concluded this lecture discussing the role of systemic therapy for the management of rare prostate cancer variants with the following take-home messages:
- Whenever possible, a metastatic biopsy should be undertaken
- For BRCA1, BRCA2, ?CDK12 (not ATM): treatment may include olaparib, rucaparib after abiraterone/enzalutamide. Ultimately, treatment will likely be olaparib, niraparib together with abiraterone/enzalutamide for front line mCRPC
- For d-MMR/MSI-high, CDK12: pembrolizumab, and checkpoint inhibitors, however, these should not replace androgen suppression inhibitors
- RB1, CDK4/6, Cyclin D: currently there is no targeted therapy, however, CDK4,6 inhibitors are under evaluation
- Primary and treatment associated small cell/neuroendocrine prostate cancer: platinum based chemotherapy
Presented by: Eric J. Small, MD, USCF Helen Diller Family Comprehensive Cancer Center, San Francisco, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022
References:
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020 May 28;382(22):2091-2102.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men with Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol 2020 Nov 10;38(32):3763-3772.


