(UroToday.com) On the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Trials in Progress Poster Session A focussed on ongoing trials that will contribute to the care of patients with prostate cancer moving forward. Dr. Morris presented the rationale and design of a phase 1 study examining JNJ-69086420, an actinium-225-labeled antibody targeting human kallikrein-2 in advanced prostate cancer.
Radioligand therapy is a rapidly developing therapeutic approach for metastatic castration resistant prostate cancer (mCRPC). Radioligand therapy has been shown to prolong survival, delay disease progression, and improve quality of life, raising hopes that these gains will be amplified with even more cancer-specific targets and more powerful radioligands. One such cancer-specific target is human kallikrein-related peptidase 2 (hK2), a tumor-associated member of the kallikrein family that shares significant homology to prostate-specific antigen and is minimally expressed in normal non-prostate tissues. JNJ‑69086420 (JNJ-420; 225Ac-DOTA-h11B6 [1,4,7,10‑tetraazacyclododecane-1,4,7,10-tetraacetic acid]), is a first-in-class radioimmunotherapy targeted to hK2 antigen. In an initial phase 0 study among men with mCRPC who progressed on standard therapies, treatment with a single dose of [111In]-DOTA-h11B6 demonstrated safety, good tumor localization, nominal normal-organ uptake, and no difference in PK between 2 and 10 mg antibody mass. Thus, the next step in the development of this clinical program is the first-in-human study to assess the safety, pharmacokinetics (PK), pharmacodynamic (PD), and clinical activity of Ac-225 radiolabeled JNJ‑420, to determine its recommended phase 2 dose (RP2D) in adults with advanced PC.
This open-label, multicenter, phase 1 study (NCT04644770) is planned to recruit approximately 50 men aged ≥18 years with advanced prostate cancer across both dose escalation (Part 1) and expansion (Part 2) parts.
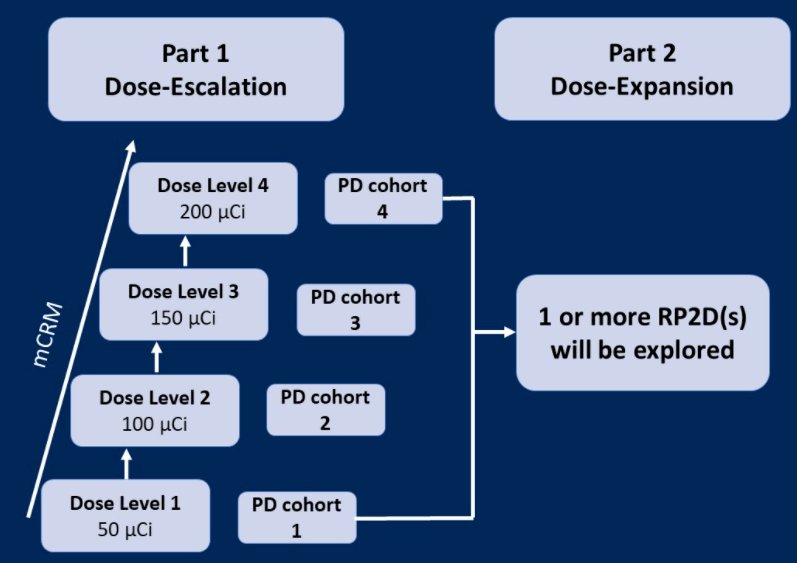
To be eligible for inclusion, men must have mCRPC with histologic confirmation of adenocarcinoma treated with at least 1 prior novel androgen receptor-targeted therapies (prior chemotherapy acceptable), Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate organ function based on hematology and serum chemistry. Patients will be excluded if they have prior treatment with radium, strontium, samarium, or radioconjugate therapy; superscan findings as protocol defined; and active central nervous system metastases.

In the dose-finding first Part, men will receive intravenous (IV) injection of 50 μCi/ 2 mg JNJ-420 (once every 8 weeks) with one or multiple doses. The escalation of dose levels will be based on dose-limiting toxicities (DLTs) evaluation until the RP2D is identified.
Subsequently, in the dose-expansion second Part, JNJ-420 will be given at one of the RP2D(s) determined in Part 1.
The primary endpoint of this study is safety, as measured by the incidence and severity [grading per NCI‑CTCAE V5.0] of AEs including DLTs, and to determine the RP2D. Secondary endpoints include prostate-specific antigen response rate and overall response rate (PCWG3 modified RECIST 1.1 criteria) as measures of preliminary clinical activity, PK, PD, immunogenicity, and biomarker analyses.
With enrollment having begun in December 2020, as of December 31 2021, 4 sites have been initiated and 19 patients enrolled. Currently, dose escalation is ongoing.
Presented by: Michael J. Morris MD, Division of Solid Tumor Oncology, Memorial Sloan Kettering Cancer Center, New York, NY


