(UroToday.com) On the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Trials in Progress Poster Session A focussed on ongoing trials that will contribute to the care of patients with prostate cancer moving forward. Dr. Mateo described the rationale and design for the ZZFIRST trial, examining enzalutamide and talazoparib for the treatment of metastatic hormone-naïve prostate cancer (mHNPC).
There is a relatively large body of evidence that DNA damage repair alterations are relatively common in patients with advanced prostate cancer. Further, significant evidence suggests that there is clinically meaningful cross talk between androgen receptor (AR) signaling pathways and DDR in prostate cancer. Thus, co-targeting of both pathways may result in a clinically relevant synergistic effect. Thus, the authors designed the ZZFIRST trial to evaluate the combination of talazoparib –a poly(ADP-ribose) polymerase inhibitor– and enzalutamide –an AR signaling inhibitor– in patients with mHNPC.
To assess this question, the authors designed a multicenter, open-label, randomized, investigator-initiated phase 2 clinical trial enrolling men aged ≥18 years with histologically confirmed mHNPC, an ECOG performance status of 0-1, and a prostate-specific antigen (PSA) ≥4 ng/mL at enrolment. Patients must have not received previous systemic treatment for locally advanced or mHNPC.
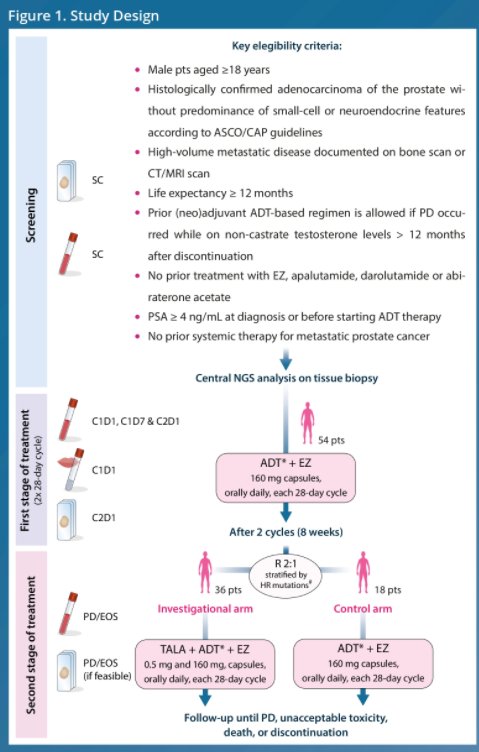
Patients will begin treatment with enzalutamide 160 mg/day for two 28-day cycles in addition to standard androgen-deprivation therapy (ADT). Following this initial lead-in period, patients are then randomized and stratified based on homologous recombination gene alterations on a 1:2 ratio to either continue enzalutamide 160 mg/day or to receive enzalutamide 160 mg/day plus talazoparib 0.5 mg/day. In both arms, patients will continue ADT throughout the trial. Treatment will continue until progressive disease or unacceptable toxicity.
In terms of assessment, PSA will be determined every 4 weeks and radiological tumor extent will be assessed at screening and every 8 weeks for the 6 initial months of treatment and every 12 weeks thereafter until progressive disease. The primary study endpoint is PSA-complete response defined as the percentage of patients with PSA < 0.2 ng/mL at 12 months of therapy. There are number of secondary and exploratory endpoints. Secondary endpoints include PSA response and time to development of castration resistant disease, the correlation between molecular and transcriptomic signatures with antitumor activity, the effect of enzalutamide and ADT on DNA repair function, and the safety and tolerability of the combination treatment approach. In terms of exploratory endpoints, the authors will assess transcriptional changes in AR and DDR pathways and assessment of genomic signatures on tumor and liquid biopsies collected at baseline, 4 weeks, and PD. An imaging sub-study of whole-body diffusion weighted MRI will help to further study antitumor activity and drug resistance mechanisms.
The authors will perform analysis using the exact binomial test. At least 11 patients must maintain PSA < 0.2 ng/mL by 12 months of therapy among 32 evaluable patients in the combination arm to justify further investigation of this strategy. A drop-out rate of 10% has been considered. This trial was opened to accrual in July 2020 at 9 sites in Spain. As of December 2021, 48 patients have been enrolled and are receiving study treatment of a targeted 54 participants.
Presented by: Joaquin Mateo MD, PhD, Vall d’Hebron Institute of Oncology (VHIO), Vall d’Hebron University Hospital


