(UroToday.com) On the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Trials in Progress Poster Session A focussed on ongoing trials that will contribute to the care of patients with prostate cancer moving forward. Dr. Sartor presented the rationale and design of the PSMAddition trial.
By way of background, [177Lu]Lu-PSMA-617 (177Lu-PSMA-617) is a high-affinity prostate-specific membrane antigen (PSMA)-targeted radioligand therapy. By binding to PSMA-expressing cells, 177Lu-PSMA-617 delivers β-particle radiation to these cells and the surrounding microenvironment. Androgen receptor pathway inhibitors (ARPI) are both indicated as the standard of care as monotherapy in patients with metastatic hormone-sensitive prostate cancer (mHSPC) and may alter PSMA expression and radiosensitivity in a potentially synergistic fashion with 177Lu-PSMA-617. PSMAddition will assess the efficacy and safety of 177Lu-PSMA-617 plus standard of care (SOC) versus SOC alone in adults with mHSPC.
The PSMAddition trial (NCT04720157) is an international, prospective, open-label, randomized, phase 3 trial in adults with mHSPC. To be eligible, patients must have PSMA-positive disease (determined by [68Ga]Ga-PSMA-11 PET/CT) and be treatment-naïve or minimally treated candidates for hormonal therapy. Further, patients are required to have an Eastern Cooperative Oncology Group performance status of 0 to 2 and adequate major organ function. Patients will be excluded if they have rapidly progressing tumors that require chemotherapy.
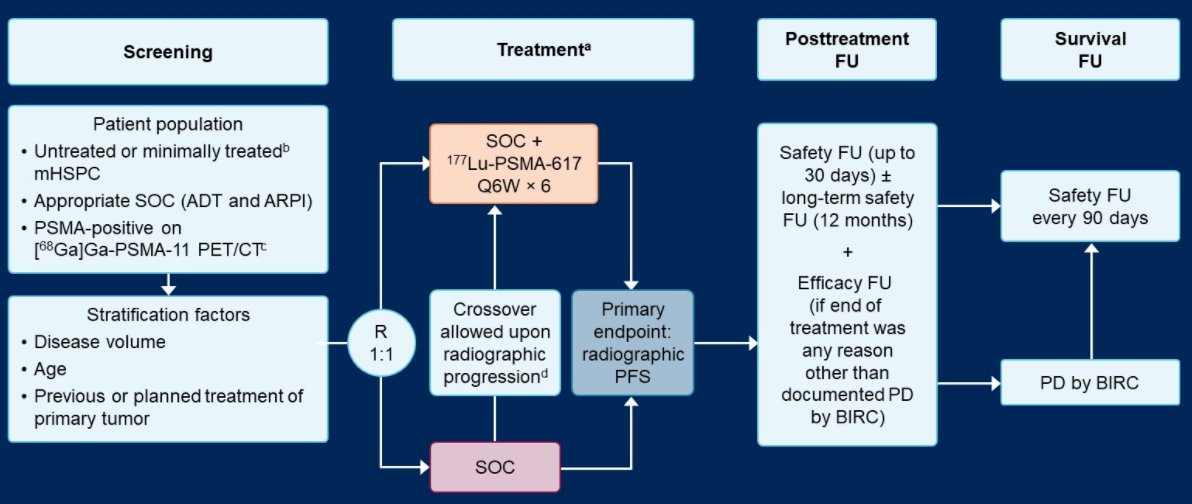
A target of approximately 1126 patients will be randomized in a 1:1 fashion to receive 177Lu-PSMA-617 (7.4 GBq i.v. every 6 weeks, ≤6 cycles) plus SOC or SOC alone (control arm). In this context, SOC is ARPI and androgen deprivation therapy. Stratification factors for randomization include tumor volume (high/low), age (≥70/ < 70 years) and previous/planned prostatectomy or radiation treatment of the primary prostate tumor (yes/no).
The primary endpoint is radiographic progression-free survival (rPFS), as assessed by blinded independent centralized review. Upon centrally confirmed radiographic progression, participants in the control arm can cross over to the 177Lu-PSMA-617 arm. The primary efficacy and safety analyses are planned for approximately 418 rPFS events which will provide 95% power to detect a hazard ratio of 0.7 for rPFS with an overall one-sided significance level of 0.025. The key secondary endpoint is overall survival, for which final analysis will occur at approximately 384 deaths. Other secondary endpoints are the proportion of patients with a prostate-specific antigen (PSA) decline of ≥90% from baseline, time to development of metastatic castration-resistant prostate cancer, composite progression-free survival (radiographic, clinical or PSA progression), safety and tolerability, and health-related quality of life.
The first patient was enrolled on trial on June 9, 2021 and currently 19 countries are participating in North America, Europe, and Asia. The estimated study completion date is December 2025.
Presented by: A. Oliver Sartor MD, School of Medicine, Tulane Medical School, New Orleans, LA


