(UroToday.com) In this presentation, Dr. Chi presented the results of the MAGNITUDE study. MAGNITUDE is a randomized phase 3 study of abiraterone acetate and prednisone (AAP) plus niraparib (NIRA) or placebo (PBO) as first-line therapy in patients with metastatic castration-resistant prostate cancer (mCRPC) with and without homologous recombination repair (HRR) gene alterations.
Up to 30% of patient with mCRPC have deleterious alterations in genes associated with HRR. These HRR alterations are associated with poor prognosis, but also confer sensitivity to PARP inhibition. Androgen receptor (AR) signaling regulates DNA repair in prostate cancer providing a rationale for combining AR targeting with PARP inhibition. NIRA is a potent PARP1/2 inhibitor that has demonstrated tumor activity in men with heavily pretreated mCRPC.1 MAGNITUDE was initiated to assess the combination of AAP with NIRA for mCRPC in prospectively selected patients with and without alterations in HRR genes.
Eligible participants had asymptomatic or mildly symptomatic treatment-naïve mCRPC, but were allowed to have received up to four months of AAP prior to enrollment. Patients were then pre-screened for HRR biomarker status. HRR positivity and negativity was defined as the presence or absence, respectively, of a deleterious alteration in one or more of the following HRR genes: ATM, BRCA1, BRCA2, BRIP1, CDK12, CHEK2, FANCA, HDAC2, or PALB2. HRR mutation status was determined on either tissue or cell-free DNA. Patients were allocated to HRR-positive or HRR-negative cohorts and then randomized to receive AAP plus NIRA or AAP plus PBO. Patients were stratified by prior taxane chemotherapy for metastatic hormone-sensitive prostate cancer (mHSPC), prior AR pathway inhibitor for non-mCRPC or mHSPC, prior AAP for first-line mCRPC, and, in the HRR-positive cohort only, BRCA1/2 versus other HRR gene alteration. The primary endpoint was radiographic progression-free survival (rPFS) by blinded independent central review (BICR). Secondary endpoints included overall survival (OS), time to cytotoxic chemotherapy, and time to symptomatic progression.
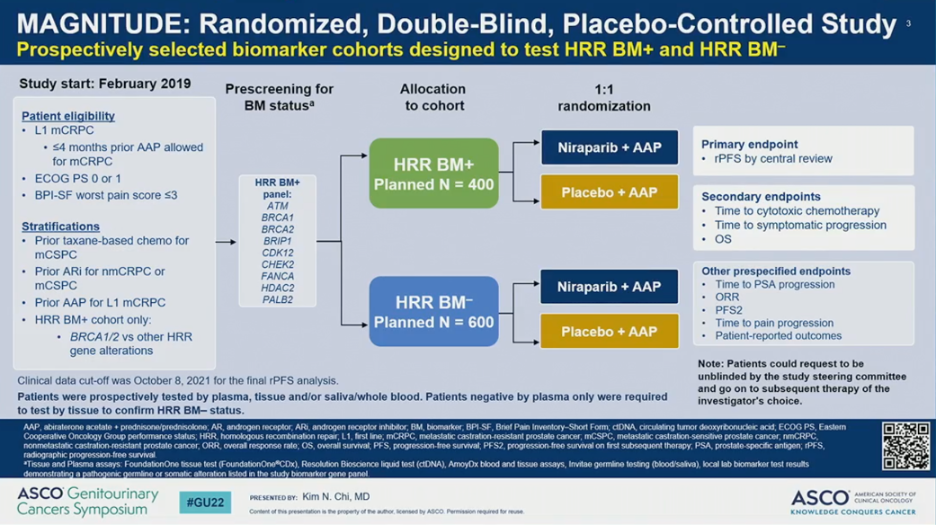
The study employed a prospective a priori approach to examining outcomes in the HRR-negative and HRR-positive cohorts. For the HRR-negative cohort, a pre-specified futility analysis was planned after enrolling 200 (of the planned 600) patients and approximately 125 composite events had occurred. The independent data monitoring committee (IDMC) was to then recommend whether the HRR-negative cohort should continue or discontinue enrollment at that time. For the HRR-positive cohort, primary and secondary endpoints were tested using a pre-specified strategy. The primary rPFS endpoint was powered for and tested first in the BRCA1/2 subgroup and if statistical significance was reached, the remainder of the HRR-positive patients would be analyzed. Approximately 50% of HRR-positive patients were specified to be BRCA1/2 positive.
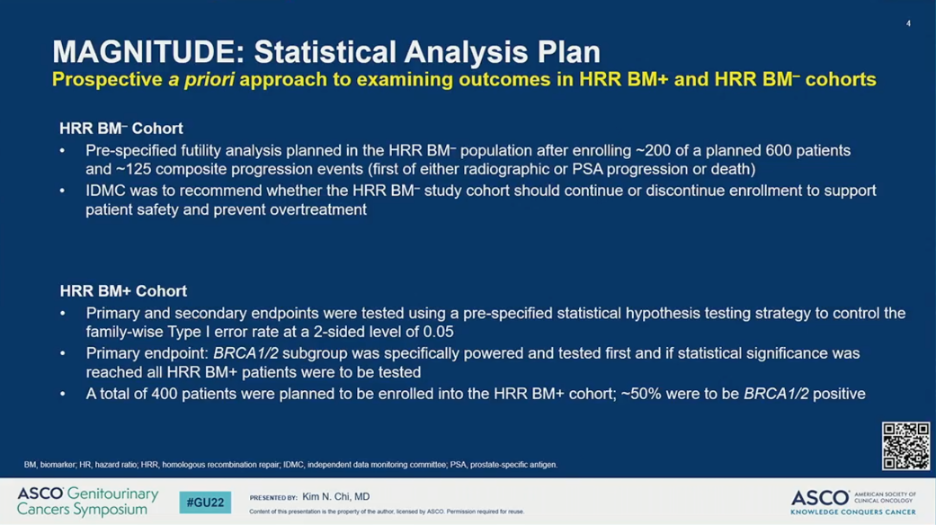
Results of the pre-specified early futility analysis in the HRR-negative cohort found no benefit for AAP plus NIRA versus AAP plus PBO. The hazard ratio for the composite endpoint (PSA or radiographic progression, whichever occurred first) was 1.09 – futility was defined as greater than 1.0. Additional grade 3 or higher toxicity was observed with the addition of NIRA. With added toxicity and no added efficacy in patients with HRR-negative mCRPC, the IDMC recommended stopping enrollment.

The HRR-positive cohort included 212 patients randomized to receive AAP plus NIRA and 211 randomized to receive AAP plus PBO. The two treatment arms were well-balanced for baseline patient characteristics including the percent with BRCA2 alterations and prior therapy. Notably, patients randomized to the NIRA arm had lower rates of ECOG PS 0 (61% versus 69%) and higher rates of visceral metastases (24% versus 19%). BRCA1 mutations were more common in the NIRA arm (5.7% versus 1.9%).

MAGNITUDE met its primary endpoint. The addition of AAP plus NIRA resulted in a significant improvement in rPFS by BICR compared to AAP plus PBO (HR = 0.53; 95% CI 0.36-0.79; P = 0.0014); this translated to a 5.7 month improvement in median rPFS. In the overall HRR-positive cohort, the addition of NIRA to AAP also resulted in a significant improvement in rPFS by BICR (HR = 0.73; 95% CI 0.56-0.96; P = 0.0217), which translated to a 2.8 month improvement in median rPFS.
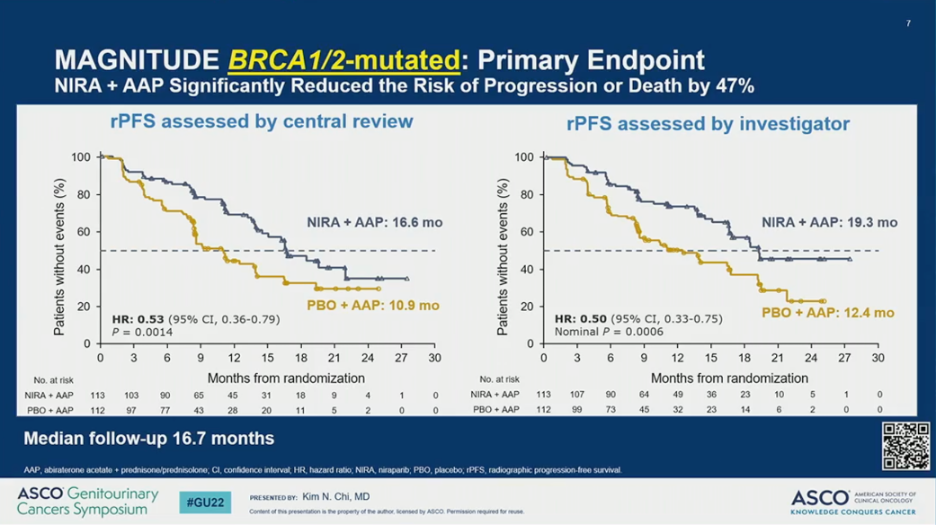
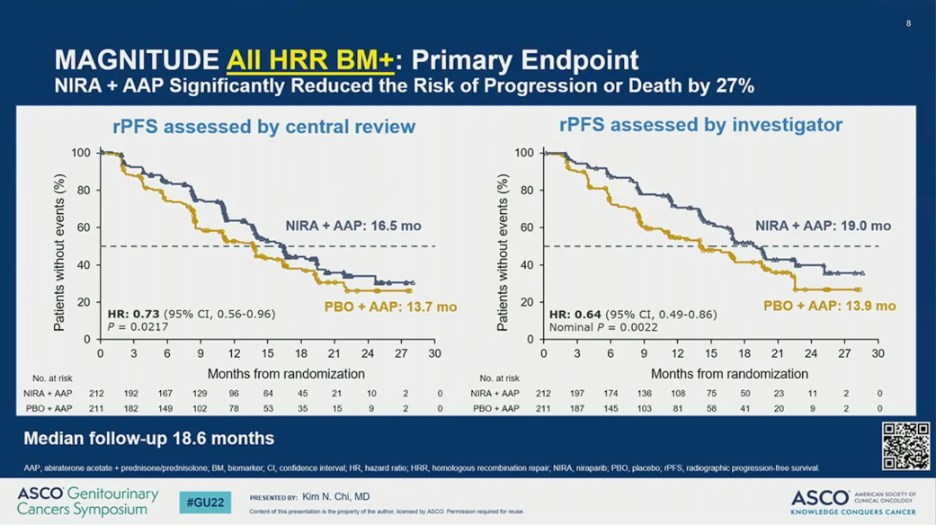
The observed rPFS benefit was consistent across subgroups. Notably, however, the HR was 0.55 (95% CI 0.38-0.81) for patients with BRCA1/2 alterations and 0.99 (95% CI 0.68-1.45) for those with other HRR mutations.
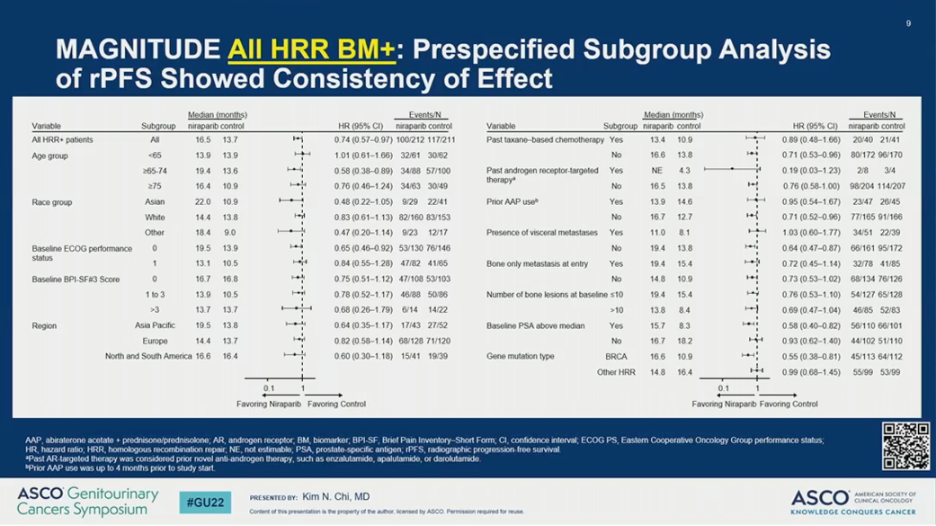
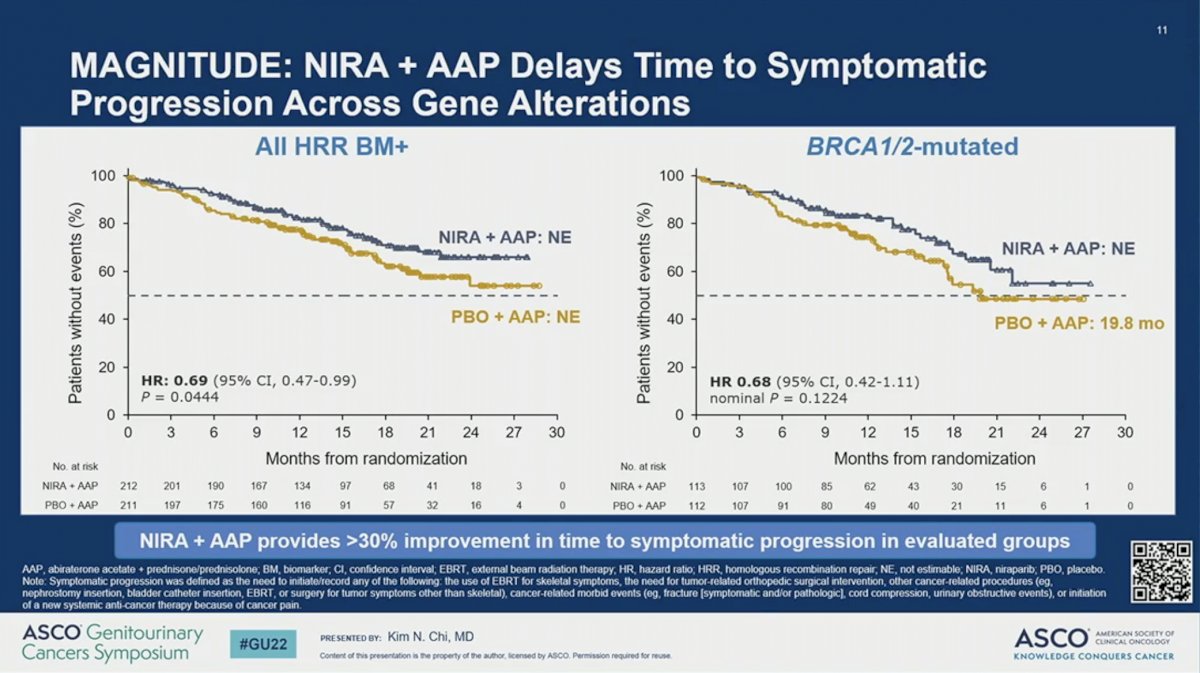
Other secondary endpoints, including time to cytotoxic chemotherapy, time to symptomatic progression, and time to PSA progression also favored the addition of NIRA to AAP for BRCA1/2-mutated and all HRR-positive patients.


The OS data is immature. Only 27% of deaths in the study population have occurred. The preliminary data suggests a trend towards improved OS with a HR of 0.94 (95% CI 0.65-1.36; P = 0.73) with the addition of NIRA to AAP in the HRR-positive cohort. A pre-specified multivariate analysis accounting for baseline characteristics showed a trend towards improved OS in patients receiving NIRA (HR = 0.77; 95% CI 0.53-1.12; P = 0.17).

The final efficacy endpoint reported, overall response rate, also favored AAP plus NIRA over AAP plus PBO in the HRR-altered (60% versus 28%) and BRCA1/2-mutated (52% versus 31%) cohort of patients. Complete response rates were also higher in for patients receiving NIRA in the HRR-altered (22% versus 11%) and the BRCA1/2-mutated (18% versus 14%) patients.

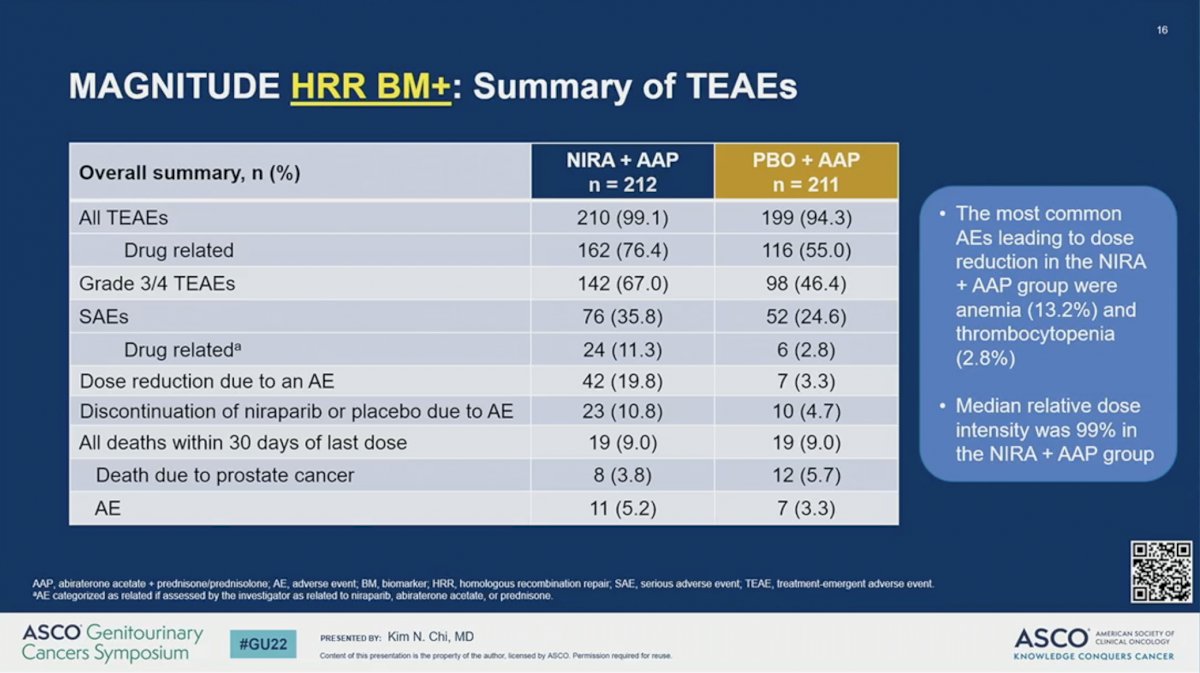
There were no new safety signals for the combination of AAP plus NIRA. The percent of patients with adverse event (AE) was higher in the NIRA than the PBO arm for all treatment-related AEs (99.1% versus 94.3%), drug-related AEs (76.4% versus 55.0%), grade 3/4 AEs (67.0% versus 46.4%), and drug-related serious AEs (11.3% versus 2.8%). Dose reductions due to an AE (19.8% versus 3.3%) and discontinuation of NIRA or PBO (10.8% versus 4.7%) were higher for those receiving NIRA than PBO. Nevertheless, the median relative dose intensity was 99% in the AAP plus NIRA arm. The most common AEs leading to dose reduction in the AAP plus NIRA group were anemia (13.2%) and thrombocytopenia (2.8%). Quality of life, as measured by the FACT-P scale, remained comparable between the two arms during the study period.

Dr. Chi concluded by summarizing that MAGNITUDE was a pragmatically designed trial to test the benefit of the combination of AAP plus NIRA for mCRPC in patients prospectively identified with and without alterations in genes associated with HRR using both tissue and blood-based approaches. No benefit was observed with the addition of NIRA to AAP in patient who were HRR-negative. AAP plus NIRA significantly improved the primary clinical outcome in HRR-positive patients with a 47% improvement in rPFS for patients with BRCA1/2 alterations and 27% across all HRR-positive patients. Future presentations showing the mature OS data and more granular analysis of the HRR-positive cohort will be important to determine which patients will benefit most from this combination treatment strategy.
Presented by: Kim N. Chi, MD, FRCPC, is a Medical Oncologist at BC Cancer – Vancouver, a Senior Research Scientist at Vancouver Prostate Centre, a Progressor of Medicine at the University of British Columbia, and Chief Medical Officer & Vice President, BC Cancer
Written by: Jacob Berchuck, MD, Genitourinary Medical Oncologist at the Dana-Farber Cancer Institute (Twitter: @jberchuck), during the 2022 ASCO Genitourinary Cancers Symposium: Thu, Feb 17, 2022 – Sat, Feb 19, 2022
References:


