(UroToday.com) In session on the third day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Poster Session C focused on Renal Cell Cancer; Adrenal, Penile, Urethral, and Testicular Cancers. In this session, Dr. Nestler discussed the role of the combination of microRNA-371a-3p and 375-5p to distinguish viable germ cell tumor and teratoma from necrosis on postchemotherapy retroperitoneal lymph node dissection (pcRPLND) specimens.
The authors assembled a cohort of 48 patients who underwent pcRPLND for patients with non-seminomatous germ cell tumor (NSGCT) who had a residual mass of 1cm or greater following chemotherapy. The authors intentionally included 16 patients each with evidence of viable tumor, teratoma, and necrosis/fibrosis. Representative regions of the pcRPLND specimens were microdissected and then total RNA was isolated and miRNA expression was analyzed for miR-371a-3p, 375-3p, and 375-5p using qPCR. ROC analysis was performed for each miRNA and for all combinations in order to determine the discriminatory capacity of viable tumor/teratoma compared to necrosis.
Comparing viable disease to necrosis, miR-371a-3p achieved the highest fold change (FC) of 31.1 (p = 0.023). In contrast, comparing teratoma to necrosis showed a that miR-375-5p performed best (FC 64,2; p < 0.001).
The authors then compared each miRNA individually and in combination to distinguish between viable disease and teratoma, compared to necrosis/fibrosis, finding that the best combination was miR-371a-3p and miR-375-5p which had an AUC of 0.94, a sensitivity of 93.75, specificity of 93.75, PPV of 96.8 and NPV of 83.3..
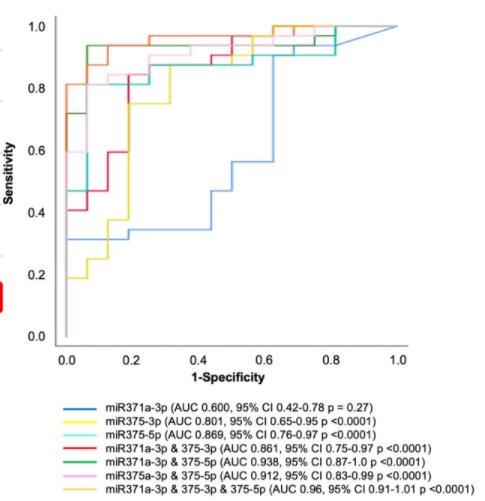
Thus, the authors conclude that miRNA samples from the pcRPLND specimen may distinguish between viable tumor or teratoma and necrosis. Moving forward, if this is validated in serum speciments, miRNA-negative patients may be spared pcRPLND.


