(UroToday.com) The 2022 GU ASCO Annual meeting included a rare tumors session featuring work from Dr. Tim Nestler and colleagues presenting results assessing differentially expressed mRNA/proteins for distinguishing viable germ cell tumors and teratomas from necrosis in retroperitoneal lymph node resections after chemotherapy. Metastatic non-seminomatous testicular tumor patients with residual retroperitoneal tumor masses > 1cm after chemotherapy are treated with post-chemotherapy RPLND. The goal of post-chemotherapy RPLND is to remove viable tumors and teratoma, which are present in approximately 10% and 40% of cases, respectively. However, histopathologically, only scar/necrosis is identified in the remaining 50% of patients:
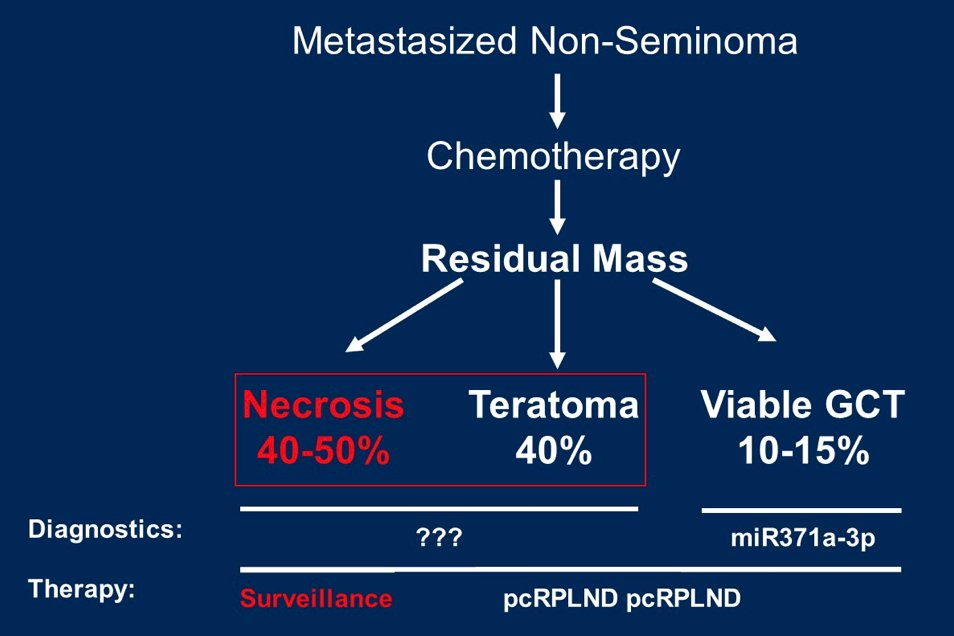
In those patients, surgical therapy is not necessary, resulting in a relevant overtreatment. So far, no adequate distinction between the histologies exists preoperatively. Recently, the first biomarker was described with miR371a-3p in serum, which is highly specific for viable tumors, but not for teratoma. Therefore, the goal is to identify mRNAs and proteins that are differentially expressed between viable tumors/teratoma vs scar/necrosis, in particular between teratoma and scar/necrosis, in post-chemotherapy RPLND resected cells.
Forty-eight patients were identified, including 16 each with teratoma/viable tumors/scar/necrosis. Representative regions of teratoma/viable tumors/scar/necrosis were microdissected and subsequently, mRNA was extracted. Initially, 770 genes were analyzed using the nCounter PanCancer Progression Panel (Nanostring). For each group comparison, genes with a fold change of < -2 / > 2 and a p-value of < 0.05 were identified. Hereafter, quantitative protein analysis (proteomics) was performed on the same samples. Finally, the proteins of the 5 mRNAs with the most different and significant expression levels between teratoma vs. scar/necrosis were validated by immunohistochemistry and H-score calculation.
By Nanostring, there were 84 significantly differentially expressed mRNAs for the group comparisons of teratoma vs. scar/necrosis, 63 for viable tumors vs. scar/necrosis, and 189 for teratoma vs. viable tumors. Quantitative protein analysis revealed 25 significantly differentially expressed proteins in teratoma vs. scar/necrosis, 254 in viable tumors vs. scar/necrosis, and 134 between teratoma vs. viable tumors. By immunohistochemistry, all 5 antibodies showed significantly increased H scores when comparing teratoma vs. scar/necrosis and teratoma vs. viable tumors. In accordance with the objective, there were two proteins identified, AGR2 and KRT19, with their corresponding genes that showed significantly differential expressions for the comparison of teratoma vs. scar/necrosis in both, quantitative protein analysis and Nanostring mRNA analysis, and were successfully validated by immunohistochemistry:
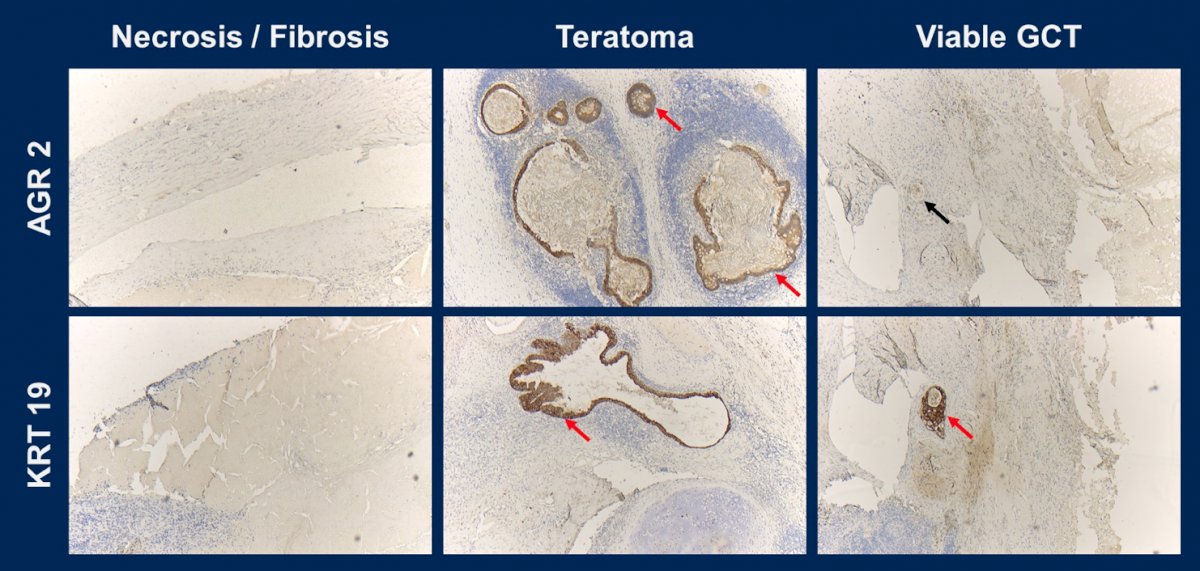
Dr. Nestler concluded this presentation by assessing differentially expressed mRNA/proteins for distinguishing viable germ cell tumors and teratomas from necrosis in retroperitoneal lymph node resections after chemotherapy with the following take-home messages:
- With AGR2 and KRT19, there are two proteins with their corresponding genes that are significantly and differentially expressed in the post-chemotherapy RPLND specimen in the clinically relevant groups teratoma vs. scar/necrosis
- Both were successfully validated by immunohistochemistry. In addition, further group differences (teratoma vs. viable tumors / viable tumors vs. scar/necrosis) were revealed depending on the analytical method
- In perspective, these proteins could be targeted by radiolabeled ligands as a tracer in order to reliably distinguish patients with teratoma from those with necrosis by means of functional imaging
- Thus, overtreatment with post-chemotherapy RPLND of patients with scar/necrosis could be safely reduced
Presented by: Tim Nestler, Department of Urology and Uro-Oncology, University Hospital of Cologne, Cologne, Germany
Co-Authors: Lara Kremer, Svenja Wagener-Ryczek, Maike Wittersheim, Melanie von Brandenstein, Pia Paffenholz, Stefan Mueller, Alexander Quaas, Martin Hellmich, Margarete Odenthal, David Pfister, Axel Heidenreich
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022


