(UroToday.com) On the third day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022 in a symposium focused on biomarkers and therapies in renal cell carcinoma (RCC), Dr. Merchan discussed the question of whether we are yet able to match sequencing report with available therapeutic options.
Dr. Merchan began by highlighting the therapeutic and genomic landscape of renal cell carcinoma over the past 30 years including the introduction followed by obsolescing of cytokine therapies, the tyrosine kinase era, and now the immune checkpoint inhibitor therapy era. From a genomic perspective, this time period encompasses characterization of the von Hippel-Lindau (VHL) gene in 1993 through to identification of angiogenic and immune gene signatures associated with response to therapy.

In the current treatment landscape, risk stratification, using the International Metastatic RCC Database Consortium (IMDC) Risk Score for RCC, encompasses clinical and laboratory features including performance status, hemoglobin, neutrophilia, thrombocytosis, hypercalcemia, and time from diagnosis to the initiation of systemic therapy. Use of these characteristics to stratify patients can importantly guide treatment selection. Dr. Merchan then posted the question of how we may integrate next generation sequencing (NGS) in this process.
Currently, most identifiable NGS findings are not immediately actionable in the context of RCC treatments. In contrast, most currently approved and utilized treatments for RCC target the tumor microenvironment and, thus far, we have little or no information on the tumor microenvironment, beyond PD-L1 status.

Across RCC subtypes, Dr. Merchan highlighted that there are a wide variety of mutations and a very diverse mutational landscape.
Based on these mutations, particularly BAP1, p53, and PBRM1, additional risk stratification may be undertaken, building on the clinical criteria in the MSKCC/Motzer criteria. This NGS enhanced risk stratification approach offers additional granularity in prognostication.

However, the real question is of treatment selection and outcome prediction, beyond prognosis. In the context of clear cell RCC, gene alterations (particularly PBRM1) are associated with treatment benefit in numerous trials including different treatment approaches including tyrosine kinase inhibitors (COMPARZ), mTOR inhibitors (RECORD-3), combination immune checkpoint inhibitor and VEGF or tyrosine kinase inhibitors (IMmotion150, IMmotion151, and JAVELIN Renal 101), or second-line immune checkpoint inhibitor monotherapy (CheckMate-025). However, interestingly, PBRM1 was not meaningfully associated with treatment benefit for patients receiving first-line dual immune checkpoint inhibition with nivolumab and ipilimumab in the CheckMate214 trial.

Liquid biopsy, comprising circulating tumor DNA (ctDNA) and cell free DNA (cfDNA), is in Dr. Merchan’s words, a promising strategy not just because of the convenience of capturing tumor genomic information from a blood draw but also capturing and accounting for RCC heterogeneity. However, to date, liquid biopsy is limited by sensitivity and the number of genes which may be examined. Thus, in his view, this approach is not ready for clinical application given the need for validation in prospective clinical trials.
However, there are a number of potentially actionable gene alterations in non-clear cell renal cell carcinoma and hereditary renal cell carcinoma syndromes. For example, in type 1 papillary RCC, the MET alterations which characterize this condition may be specifically actionable using MET inhibitors. Further, in patients with FH mutations in papillary RCC (including in particular in HLRCC) erlotinib and bevacizumab may be particularly well suited. Additionally, in TSC, mTOR inhibitor based therapies are mechanistically most appropriate while the novel HIF-2alpha inhibitor belzutifan is being evaluated in VHL syndrome related clear cell RCC.
As highlighted above, tumor microenvironment signatures may be better suited to actionability than genomic alterations. A number of different approaches have been explored including ccRCC molecular subtypes (examined in the BIONNIK trial), the IMmotion150 strata (Angio, Teff/IFNγ, and myeloid/inflammatory subgroups), and the JAVELIN Renal 101 signature. Moving forward, he emphasized the transition from genome to transcriptome to clinical trials. To this end, he highlighted a proposed phase II, open-label, parallel single-arm study using tumor RNAseq cluster to assign protocol treatments.
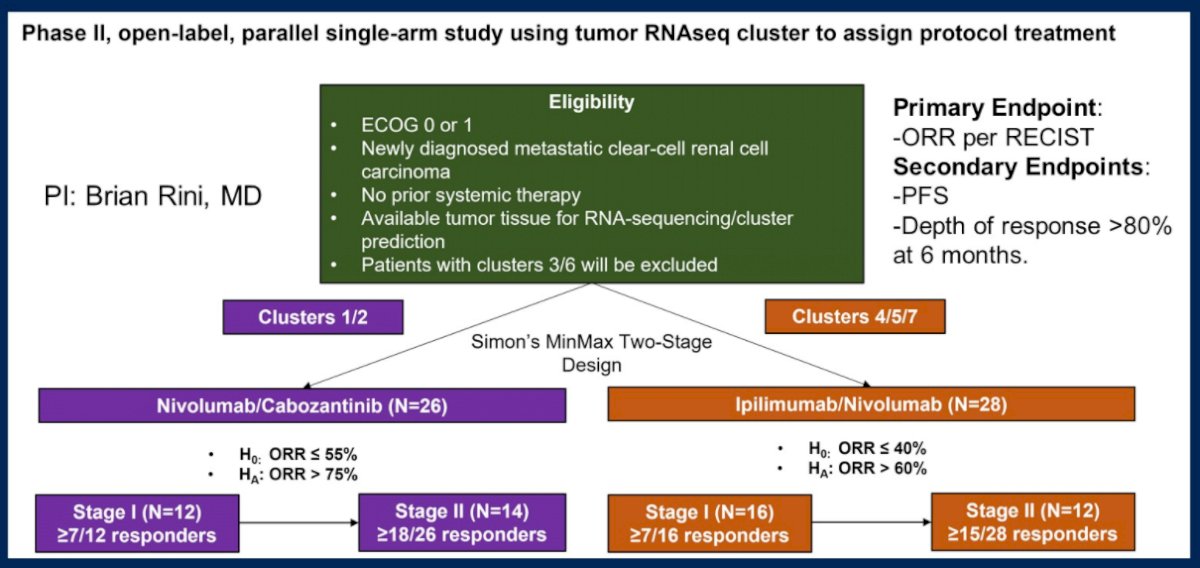
In conclusion, he emphasized that the field of RCC genomics and transcriptomics is rapidly evolving. While there is no level 1 evidence to support the use of NGS to guide treatment in RCC, we currently utilize clinical criteria to guide clinical care. Further studies prospectively integrating clinical, genomic, and transcriptomic data with treatment and outcomes will be necessary to identify markers to guide treatment decisions.
Presented by: Jaime R. Merchan, MD, MMedSc, University of Miami-Miller School of Medicine, Sylvester Comprehensive Cancer Center

