(UroToday.com) In the Poster Session C on the third day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022 focused on Renal Cell Cancer; Adrenal, Penile, Urethral, and Testicular Cancers. In this session, Dr. Rha presented subgroup analyses of efficacy and safety from the CLEAR trial of Lenvatinib and pembrolizumab among East Asian patients with advanced renal cell carcinoma (aRCC).
The phase 3 CLEAR trial demonstrated that Lenvatinib and pembrolizumab resulted in significant improvements in progression-free survival (PFS; hazard ratio [HR] 0.39; 95% CI 0.32, 0.49; P < 0.001), overall survival (OS; HR 0.66; 95% CI 0.49, 0.88; P = 0.005) and objective response rate (ORR; odds ratio 4.35; 95% CI 3.16, 5.97) compared to sunitinib. In this analysis, the authors report subgroup analyses among patients from Japan and the Republic of Korea.
While previously described and published, briefly, the phase 3 CLEAR trial enrolled patients with aRCC and no prior systemic therapy and randomized them in a 1:1:1 fashion to receive 1 of 3 treatments including lenvatinib 20 mg PO QD and pembrolizumab 200 mg IV Q3W and sunitinib 50 mg PO QD (4 weeks on/2 weeks off). Randomization was stratified by geographic region and MSKCC prognostic groups. The primary endpoint was PFS; secondary endpoints included OS, ORR and safety. An independent review committee assessed tumors per RECIST v1.1. Median PFS and OS were calculated using the Kaplan-Meier method; HR and 95% CI were estimated by a stratified Cox model. Odds ratios were estimated by a stratified Cochran-Mantel-Haenszel test. This analysis compares the efficacy and safety of LEN + PEMBRO vs SUN in the East Asian subset of the CLEAR trial including patients from Japan and the Republic of Korea.
Among 1069 patients randomized in the CLEAR trial, 75 patients in the Lenvatinib and pembrolizumab group and 65 patients in the sunitinib group were from East Asia. The baseline characteristics of patients from East Asia were comparable to the overall CLEAR study population and generally consistent between the treatment groups. Progression-free survival was significantly longer among patients who received Lenvatinib and pembrolizumab, compared to sunitinib (median 22.1 vs 11.1 months; HR 0.38, 95% CI 0.23, 0.62) as was overall survival (HR 0.71, 95% CI 0.30, 1.71) though median OS was not reached for both arms.

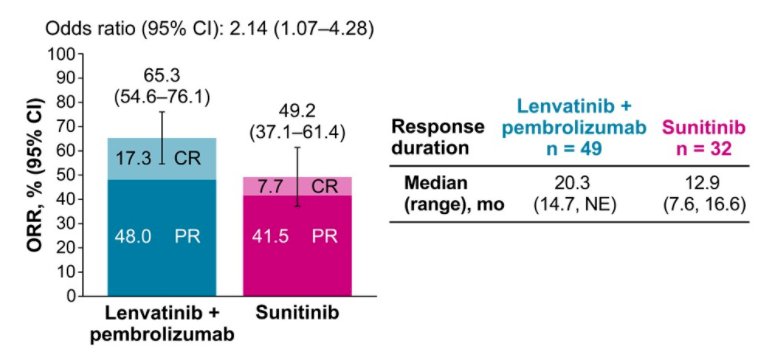
In keeping with the overall population, ORR was improved with Lenvatinib and pembrolizumab, compared to sunitinib (65.3% vs 49.2%; odds ratio 2.14, 95% CI 1.07, 4.28). Additionally, the duration of response was longer for patients receiving Lenvatinib and pembrolizumab
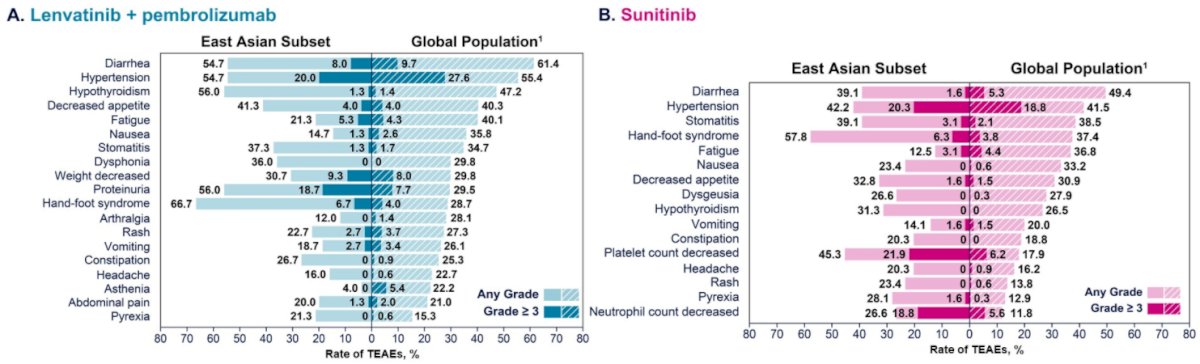
Grade ≥3 treatment emergent adverse events (TEAEs) occurred in 88.0% of patients in the Lenvatinib and pembrolizumab group and in 79.7% of patients in the sunitinib group. The incidences and types of TEAEs were generally similar to the overall CLEAR population and were manageable with dose adjustments and appropriate concomitant therapies.
The authors therefore conclude that this subset of East Asian patient demonstrated consistent results with the overall CLEAR study population and support the use of Lenvatinib and pembrolizumab as first-line therapy in mRCC.
Presented by: Sun Young Rha MD, PhD, Yonsei Cancer Center, Yonsei University Health System, Seoul, South Korea


