(UroToday.com) In a poster presentation on the third day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022 focussed on Renal Cell Cancer; Adrenal, Penile, Urethral, and Testicular Cancers, Dr. Ged presented the rationale and design of the ORCHID trial, examining olaparib in patients with metastatic renal cell carcinoma (mRCC) with DDR mutations.
Dr. Yasser Ged first emphasized that mRCC comprises a wide set of histologically and biologically heterogeneous tumors, many of which are increasingly recognized to harbour alterations that affect genomic instability, including DNA repair pathways. This observation formed the basis of recent in vitro studies demonstrating that RCC cells are sensitive to PARP inhibitors, with subsequently increased DNA replication stress, DNA synthesis suppression, and eventually increased cell cycle arrest. Further potentiating this mechanism, following conditional knockout of BAP-1 was associated with reduced repair of double stranded DNA (dsDNA) breaks suggesting that PARP inhibitors may result in synthetic lethality in the setting of BAP-1 inactivation.
The ORCHID trial is an open-label, single-arm, investigator-initiated phase II trial of olaparib in patients with metastatic RCC (NCT03786796). To be eligible for inclusion, patients must be adults age 18 years or older with histologically confirmed, unresectable, locally advanced, or mRCC who have had disease progression after at least one VEGF targeted therapy or immune checkpoint inhibitor and have evidence of deleterious somatic or germline DDR gene alteration (BAP1, ATM, BRCA1, BRCA2, PALB2, CHEK2, BRIP1, RAD51C, BARD1, CDK12, CHEK1, FANCL, PP2R2A, RAD51B, RAD51D, or RAD54L).
Patients will be treated with olaparib at an initial dose of 150mg by mouth twice daily. If olaparib is tolerated without any grade > 3 adverse events after one month, the dose will be increased to the FDA-approved dose of 300mg by mouth twice daily. Patients will be followed monthly with clinic visits, safety labs, and toxicity assessments. Treatment will be continued until radiographic progression (RECIST version 1.1) or unmanageable toxicity requiring drug cessation.
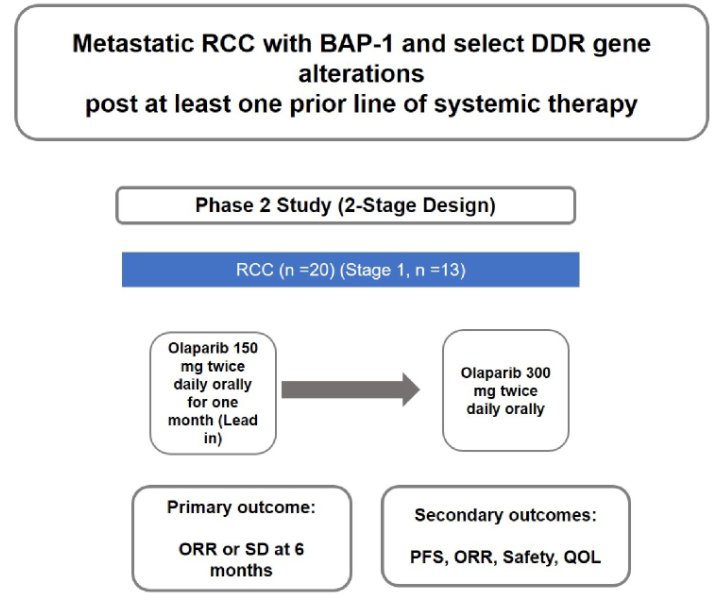
The authors aim to enroll 20 patients to evaluate the primary endpoint of disease control rate (defined as complete response [CR], partial response [PR], or stable disease [SD]) at six months of treatment) according to RECIST version 1.1. The authors use Simon’s two-stage minimax design to test the null hypothesis of a 5% disease control rate versus the alternate of 25%. They will further assess secondary endpoints including progression-free survival, best overall response, and safety. The study is currently open to enrollment at Johns Hopkins.
Presented by: Yasser Ged, MRCP, MBBS, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, MD

