(UroToday.com) The 2022 GU ASCO Annual meeting included a urothelial carcinoma session highlighting work from Dr. Irene Tsung and colleagues presenting results of ABLE, a phase 2, single-arm, two-stage study of nab-paclitaxel with anti-PD1/PDL1 in advanced urothelial cancer. Anti-PD/PDL1 immune checkpoint inhibitor monotherapy is standard in select PDL1 expressing advanced urothelial cancer and platinum-refractory advanced urothelial cancer. Nab-paclitaxel previously showed encouraging activity in platinum-refractory advanced urothelial cancer. Dr. Tsung and investigators conducted a single-arm trial of the combination of nab-paclitaxel and pembrolizumab in platinum-refractory or cisplatin-ineligible advanced urothelial cancer.
Eligible patients had RECIST 1.1 measurable urothelial cancer, grade ≤1 neuropathy, and ECOG PS 0-2. Study therapy consisted of nab-paclitaxel at starting dose of 125 mg/m2 IV on days 1 and 8 and pembrolizumab 200 mg IV on day 1 in 21-day cycles until progression, intolerable toxicity, death, or consent withdrawal. Continuing nab-paclitaxel after 6 cycles were optional. Nab-paclitaxel starting dose was reduced to 100 mg/m2 after the planned interim analysis on the first 17 subjects. The study design for ABLE is as follows:
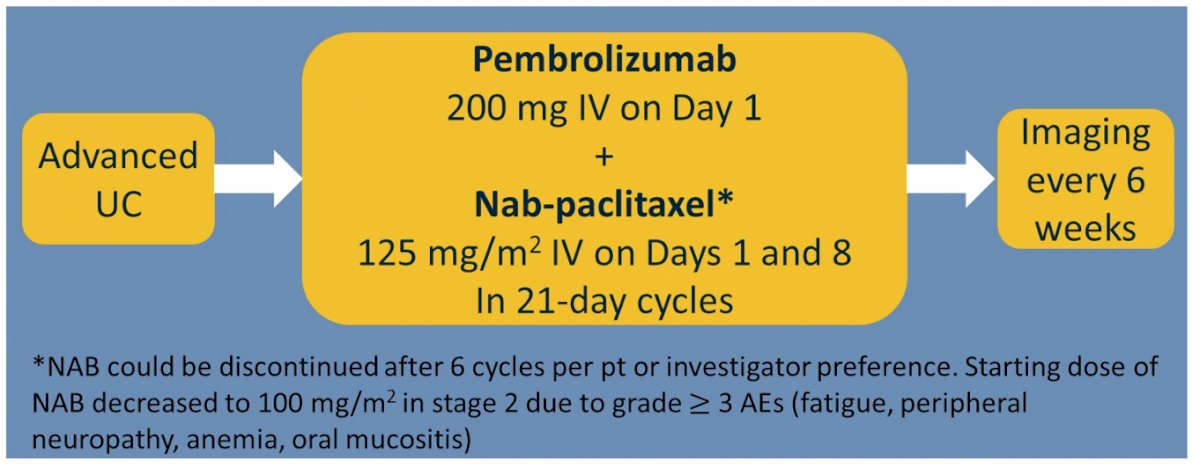
The primary endpoint was overall response rate (ORR) by RECIST 1.1, and secondary endpoints included safety/toxicity, progression free survival (PFS), overall survival, complete response proportion, duration of response (DOR), and duration of therapy.
Between February 2018 and April 2021, 36 response evaluable patients were enrolled, including 11 with tumors of upper tract origin, 32 men, mean age 71.5 years (range 52 – 88), 25 pure urothelial histology, 15 with platinum-refractory, 21 cisplatin-ineligible by Galsky criteria, and ECOG performance status was 0, 1 or 2 in 9, 20, and 7 patients, respectively. Unconfirmed best ORR was 58.3% (95% CI 42-74) including 3 CR and 18 PR, confirmed ORR 50% (18/36); 31/36 patients experienced some tumor shrinkage:

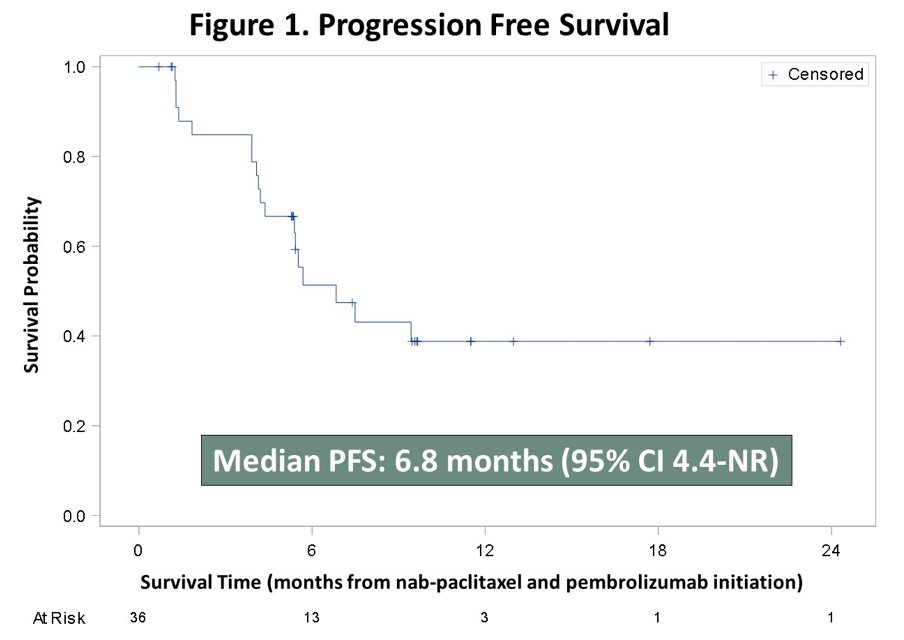
Median DOR was 19 weeks (95% CI 15.6-34.8), and median PFS 6.8 months (95% CI 4.4-not reached):
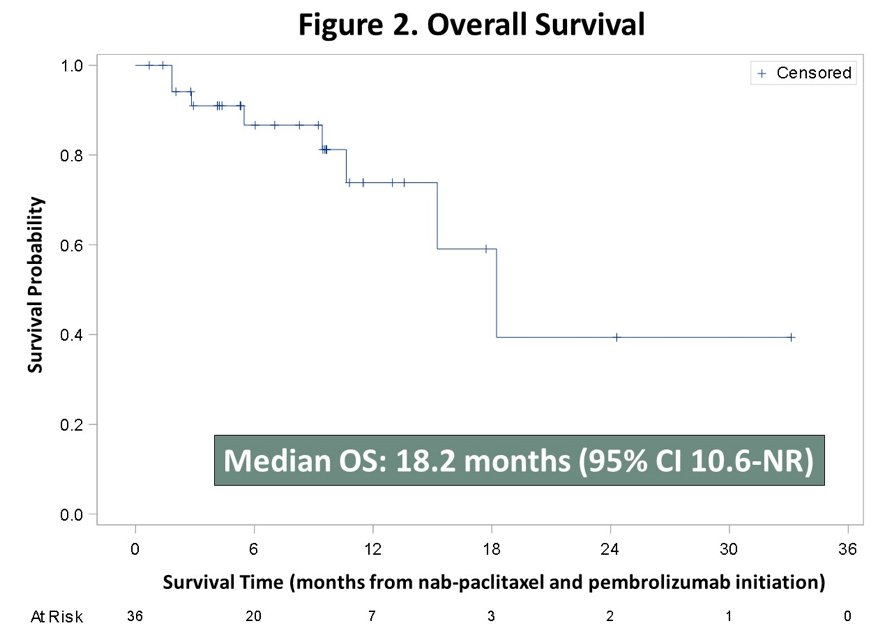
The median overall survival was 18.2 months (95% CI 10.6-not reached):
Patients received a median of 6 cycles (range 1-14) with median duration of therapy of 4.2 months (range: 0.6-9.6). Grade ≥3 adverse events occurred in 25 patients including fatigue (n = 6), anemia (n = 6), peripheral neuropathy (n = 3), and oral mucositis (n = 3); there were 6 patients that discontinued treatment due to adverse events. Ten patients had immune mediated adverse events, including one with encephalitis. Archival tumor NGS revealed TMB ≥10 in 5/21 available specimens.
Dr. Tsung concluded her presentation of the ABLE trial noting that the combination of nab-paclitaxel and pembrolizumab had reasonable toxicity profile with promising response rates in advanced urothelial cancer patients who cannot receive cisplatin or are platinum-refractory.
Clinical trial information: NCT03240016.
Presented by: Irene Tsung, MD, University of Michigan Health System, Ann Arbor, MICo-Authors: Edward Green, Phillip Lee Palmbos, Zachery R Reichert, Ulka N. Vaishampayan, David C. Smith, Megan Veresh Caram, Sarah Elizabeth Yentz, Stephanie Daignault-Newton, Zachery Sloan, Laura Hurley, Ajjai Shivaram Alva
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022


