(UroToday.com) The 2022 GU ASCO Annual meeting included a urothelial carcinoma session featuring work from Dr. Li Zhou and colleagues presenting the preliminary results of the RC48-C014 trial assessing RC48-ADC combined with toripalimab in patients with locally advanced or metastatic urothelial carcinoma. RC48-ADC is a novel humanized anti-HER2 antibody-drug conjugate, which showed promising data in HER2-positive and even negative patients with metastatic urothelial carcinoma. In the second line treatment of HER2 overexpressed locally advanced or metastatic urothelial carcinoma, RC48-ADC showed an ORR of 51.2%, PFS of 6.9 months, and OS of 13.9 months. Toripalimab is an anti-PD-1 antibody with a durable antitumor effect for metastatic urothelial carcinoma (ORR of 26%, PFS of 2.3 months, and OS of 14.4 months). The combination may have a synergistic antitumor effect. Initial RC48-C014 data was previously presented at ASCO 2021, and at GU ASCO 2022 Dr. Zhou and investigators report an update on safety and ORR.
In dose-escalation cohort, patients received 1.5 or 2 mg/kg RC48-ADC + 3mg/kg toripalimab with the traditional 3+3 escalation design. In the expansion cohort, patients received the recommended dose of RC48-ADC + toripalimab every 2 weeks. The primary endpoints were safety/tolerability and recommended RC48-ADC dose, and secondary endpoints included pharmacokinetics, ORR per RECIST 1.1, PFS, and OS, stratified by HER2 and PD-L1 expression.
As of September 23, 2021 (data cutoff), 32 metastatic urothelial carcinoma patients (18 males; median age 67 years [range: 52-76]) were enrolled since August 20, 2020. Overall, 53% of patients were systemic treatment naïve in the locally advanced or metastatic setting. The primary site was in upper tract urothelial carcinoma in 56%, and 53% had visceral metastases, including 28% with liver metastases. HER2 expression was positive (IHC 3+ or 2+ ISH+) in 19% of patients, and PD-L1 CPS≥1 in 56%. No dose-limiting toxicity was reported, and the recommended dose for RC48-ADC was 2mg/kg. At data cutoff, confirmed investigator-assessed ORR was 75% (95% CI 50.9, 91.3), including 15% CRs; disease control rate was 95% (95% CI 75.1, 99.9). The ORR for first-line previously untreated metastatic urothelial carcinoma patients was 80% and 75% for patients with liver metastases. The ORR was 100% for patients with HER2 (3+), 77.8% for HER2 (2+), 66.7% for HER2 (1+), and 50% for HER2 (0), respectively. The ORR was 97.1% in patients with PD-L1 CPS≥1 and 50% in CPS < 1. The time to response and duration of response is as follows:
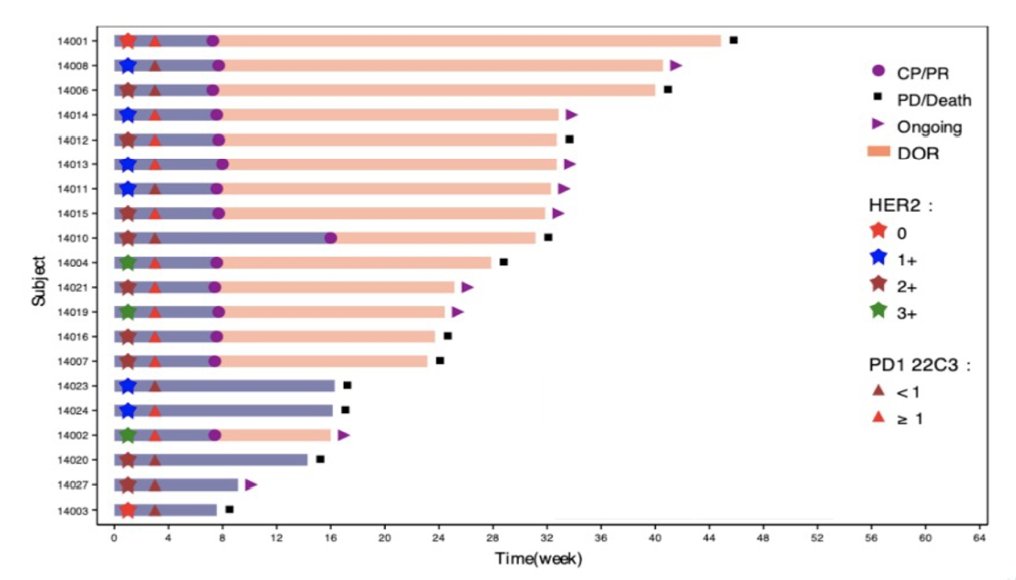
The percent change from baseline in sum of diameters of target lesions, stratified by PR/CR and nonresponders is as follows:
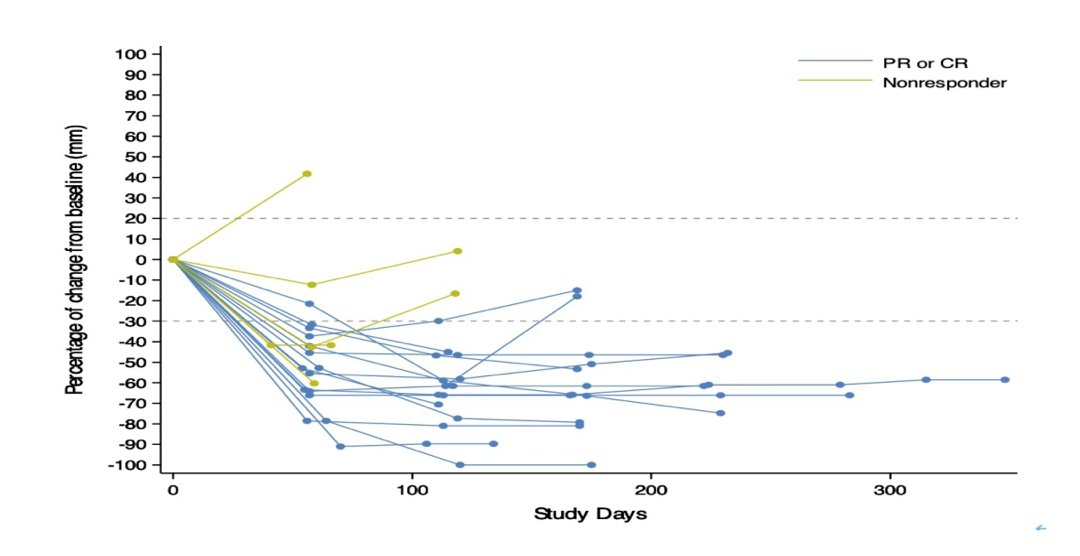
The most common treatment-related adverse events were anorexia (72%), asthenia (56%, 8%≥G3), aminotransferase level increased (56%, 4%≥G3), peripheral sensory neuropathy (56%), alopecia (52%), nausea (36%), and anemia (32%). The most common immune-related adverse event was pneumonitis (20%). Follow-up continues for PFS and OS.
Dr. Zhou concluded this presentation of the preliminary results of the RC48-C014 trial assessing RC48-ADC combined with toripalimab in patients with locally advanced or metastatic urothelial carcinoma with the following take-home messages:
- RC48-ADC in combination with toripalimab demonstrated promising efficacy (ORR of 75% in all patients) in patients with metastatic urothelial carcinoma and a manageable safety profile
- The assessment of RC48 + toripalimab vs platinum-based chemotherapy in treatment naive metastatic urothelial carcinoma patients is being planned
Presented by: Li Zhou, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Genitourinary Oncology, Peking University Cancer Hospital & Institute, Beijing, China
Co-Authors: Huayan Xu, Siming Li, Xieqiao Yan, Juan Li, Xiaowen Wu, Zhihong Chi, Lu Si, Chuanliang Cui, Yan Kong, Bixia Tang, Lili Mao, Bin Lian, Xue Bai, Xuan Wang, Hongqian Guo, Zhisong He, Jun Guo, Xinan Sheng
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022


