(UroToday.com) On the second day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Dr. Rose presented in a session highlighting novel therapies in bladder cancer and their toxicities, discussing the future of adjuvant and neoadjuvant therapy for patients with muscle-invasive bladder cancer (MIBC).
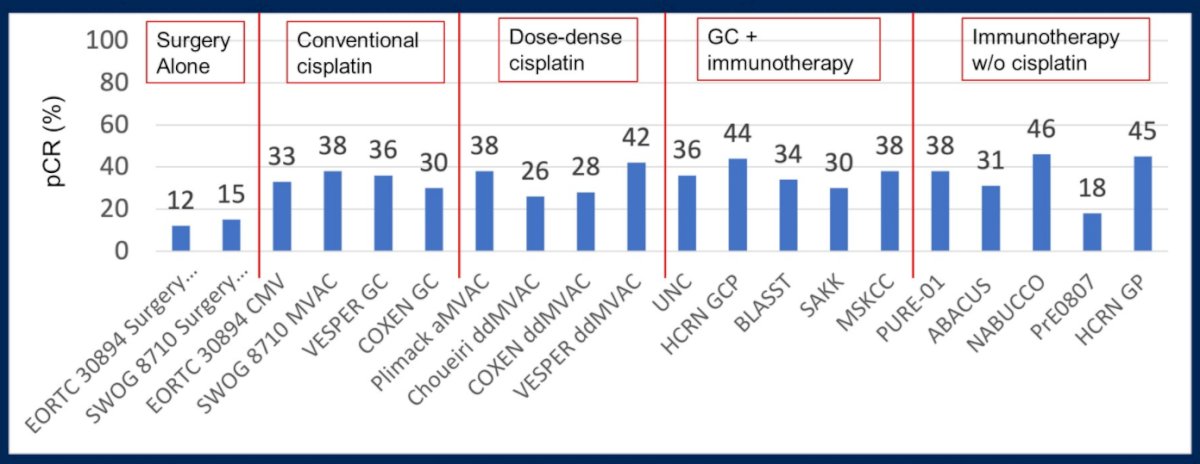
Dr. Rose began by emphasizing that neoadjuvant cisplatin-based combination chemotherapy prior to radical cystectomy is the standard of care for patients with MIBC, based on data from SWOG-8710 and BA06 30894. Cisplatin-based chemotherapy which results in a pathologic complete response at the time of radical cystectomy is importantly associated with a favourable long-term prognosis. In the two aforementioned trials, pathological complete response rates were 20-23% higher among those patients who receive neoadjuvant chemotherapy. As highlighted in the figure below from the SWOG trial, this translated into meaningful differences in long-term survival.
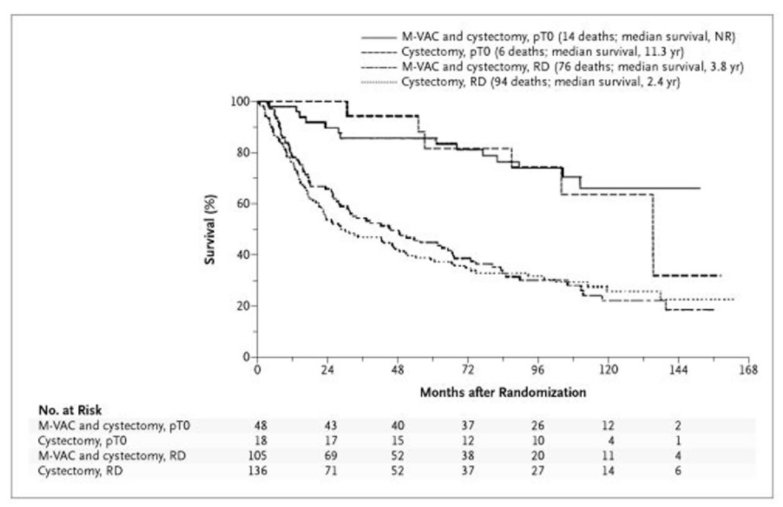
Further, data from VESPER presented at ESMO 2021, have demonstrated that dose-dense regimes (dose dense MVAC) have numerically higher rates of pathological downstaging than gemcitabine-cisplatin. This translates into improved three-year progression-free survival (66% vs 56%; hazard ratio 0.70, 95% CI 0.51-0.96). While these dose-dense regimes are clearly highly active in the neoadjuvant setting, Dr. Rose emphasized that they don’t dramatically improve pathological complete response rates compared with conventional cisplatin-based regimes.
Moving onwards to future considerations in this disease space, Dr. Rose highlighted that trials of chemo-immunotherapy in patients with metastatic urothelial carcinoma, such as KEYNOTE-361, have shown modest if any improvements in outcomes and this approach has not been adopted. However, several single armed studies, of which she highlighted five, have shown a high response rate of chemo-immunotherapy in MIBC in the neoadjuvant space, with pathological complete response rates of 30-44% and downstaging to non-muscle invasive disease in 50-66%.
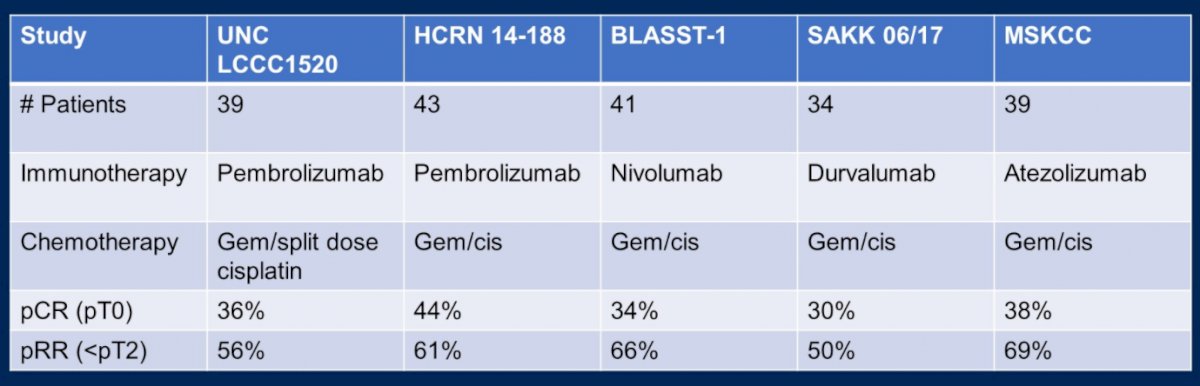
However, the ability to achieve a pathological response rate in the majority of patients remains elusive, whether using conventional chemotherapy regimes (30-38% pCR rate), dose-dense cisplatin regimes (26-42% pCR rate), or chemo-immunotherapy regimes (30-44% pCR rates). However, Dr. Rose highlighted several large, ongoing randomized trial investigating combination chemo-immunotherapy in MIBC, including NIAGARA (combining durvalumab with gemcitabine-cisplatin), KEYNOTE-866 (combining pembrolizumb with gemcitabine-cisplatin), and ENERGIZE (combining nivolumab or nivolumab and the IDO1-inhibitor linrodostat with gemcitabine-cisplatin). In each case, the studies are designed to assess the co-primary endpoints of pathological complete response and event free survival. Further, each trial offers adjuvant therapy in the arms receiving combined chemo-immunotherapy.
However, considering the potential forthcoming results of these trials, Dr. Rose raised a number of questions, assuming that these studies are positive. First, they will not answer whether this combined neoadjuvant chemo-immunotherapy and adjuvant immunotherapy approach is superior to neoadjuvant chemotherapy followed by adjuvant immunotherapy. Or, whether neoadjuvant immunotherapy alone followed by adjuvant chemotherapy may be comparable. Further, there is the ongoing question of whether we can identify patients with pathological complete response prior to surgery and spare them cystectomy. Additionally, she raised the question of whether these trials endpoints are relevant and acceptable. Finally, she emphasized the importance of considering the treatment options available to the significant proportion of patients who are not able to receive cisplatin.
To this end, immunotherapy without chemotherapy is also being investigated in the neoadjuvant setting. Across five trials, she highlighted “impressive” initial results including pathological complete response rates ranging up to 46%.
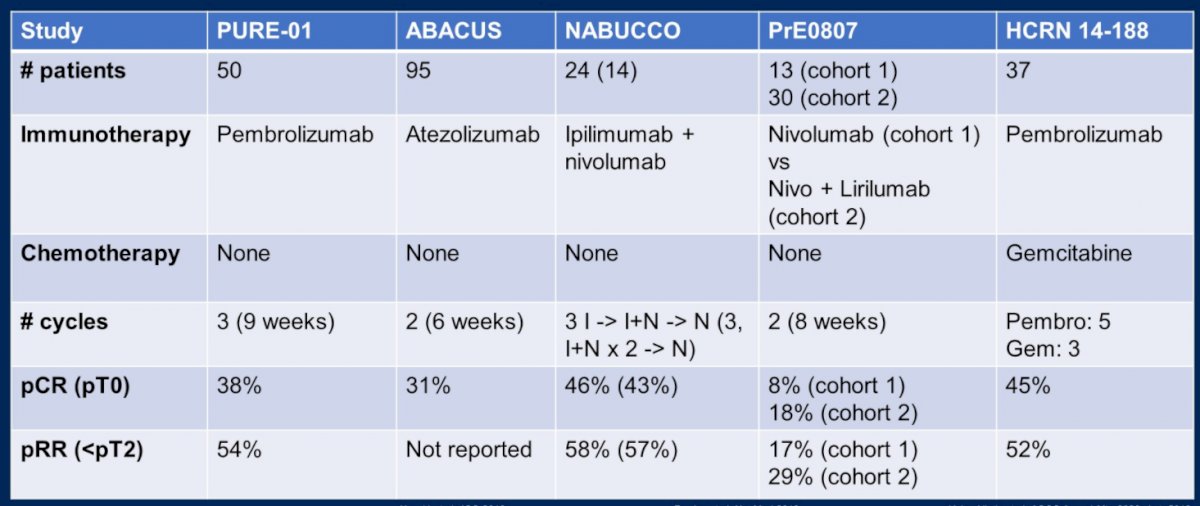
However, she emphasized that it is impossible to compare outcomes across the large number of small single arm trials in this disease space. Thus, ongoing randomized trials will be critical to better understand the role of each of these treatment approaches.
Moving beyond chemotherapy and immune checkpoint inhibitors, Dr. Rose highlighted that enfortumab vedotin is being extensively investigated as a peri-operative treatment in patients with MIBC, including those who are cisplatin-eligible (in EV-304/KEYNOTE-B15) and cisplatin-ineligible (VOLGA, EV-103 cohort H, EV-103 cohort J, and EV-303/KEYNOTE-905).
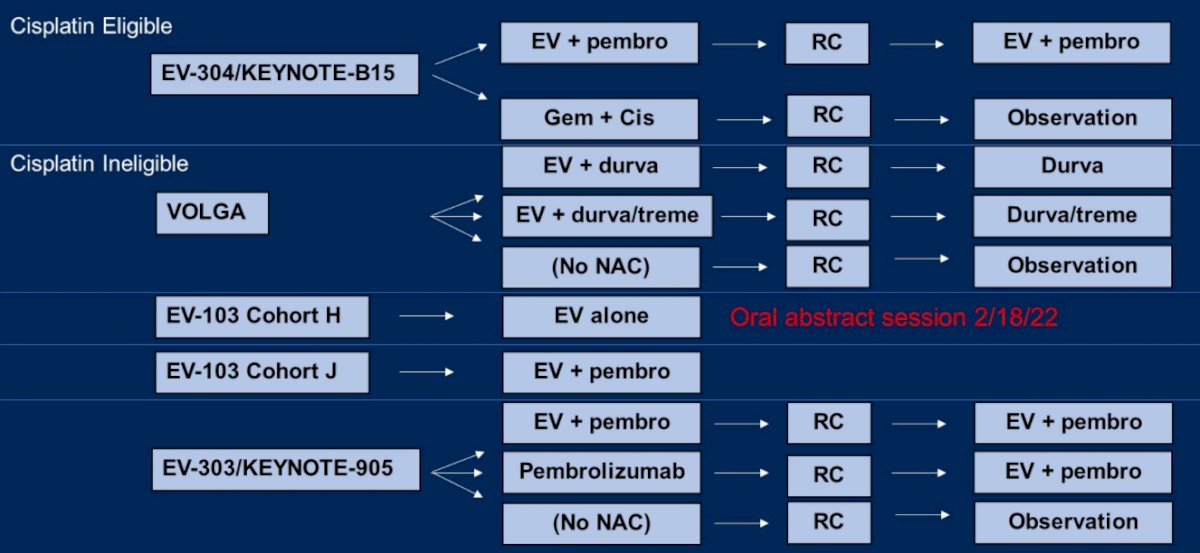
Looking forward five years, she considered a number of potential standards of care for patients who are cisplatin-ineligible who are undergoing radical cystectomy including surgery alone (the most widely used approach today), neoadjuvant immune checkpoint inhibition, neoadjuvant enfortumab vedotin, or a combination of these agents.
Building off these ideas, she emphasized the question of whether, if we can improve pathologic complete response, we can omit radical cystectomy with bladder-sparing approaches. This is actively being tested in the RETAIN-2 and A031701 trials which are ongoing.
She further considered the question of whether immunotherapy should be given pre-operatively or post-operatively. The phase III CheckMate274 trial, published June 2021, showed a benefit to adjuvant nivolumab following surgery for patients with residual muscle invasive disease or node positive disease following neoadjuvant chemotherapy. In contrast, IMvigor010 failed to show an improvement in disease-free survival with adjuvant atezolizumab in the intention-to-treat population, though subsequent analyses demonstrated a benefit among patients with detectable circulating tumor DNA.
Thus, in conclusion, Dr. Rose highlighted that cisplatin-based combination chemotherapy, while our current approach, may be a temporary standard of care. A pathologic complete response for the majority of patients remains elusive though may be important to move the goal of bladder sparing forward. Immunotherapy is now established in the adjuvant setting and is under active investigation in the neoadjuvant space.
Presented by: Tracy L Rose, MD, The University of North Carolina at Chapel Hill and Lineberger Comprehensive Cancer Center

