(UroToday.com) The 2022 GU ASCO Annual meeting included a urothelial carcinoma trials in progress session featuring BOND3, a phase 3, single-arm study of CG0070 in subjects with nonmuscle invasive bladder cancer (NMIBC) unresponsive to BCG, presented by Dr. Edward Uchio. CG0070 is a serotype 5 adenovirus engineered to express GM-CSF and replicate in cells with mutated or deficient RB:

Phase I and II trials have reported response rates (RR) of approximately 45% observed in patients with recurrent NMIBC after BCG [1-2]. BOND3, a single arm phase 3 study (NCT04452591), was launched to confirm the clinical activity of CG0070 in patients with BCG unresponsive NMIBC.
A total of 110 patients with BCG-unresponsive CIS with or without concurrent Ta or T1 disease will be treated with intravesical CG0070 at a dose of 1x1012 vp. CG0070 will be administered as follows: induction weekly x 6 followed by weekly x 3 maintenance instillations at months 3, 6, 9, 12, and 18. Patients with persistent CIS or HG Ta at 3 months may receive re-induction with weekly x 6 CG0070. Assessment of response will include every 3 month cystoscopy with biopsy of areas suspicious for disease, urine cytology, CTU/MRU, and mandatory bladder mapping at 12 months. Detection of high grade disease within the bladder will be enumerated as recurrence or non-response. The study administration schedule for BOND3 is as follows:
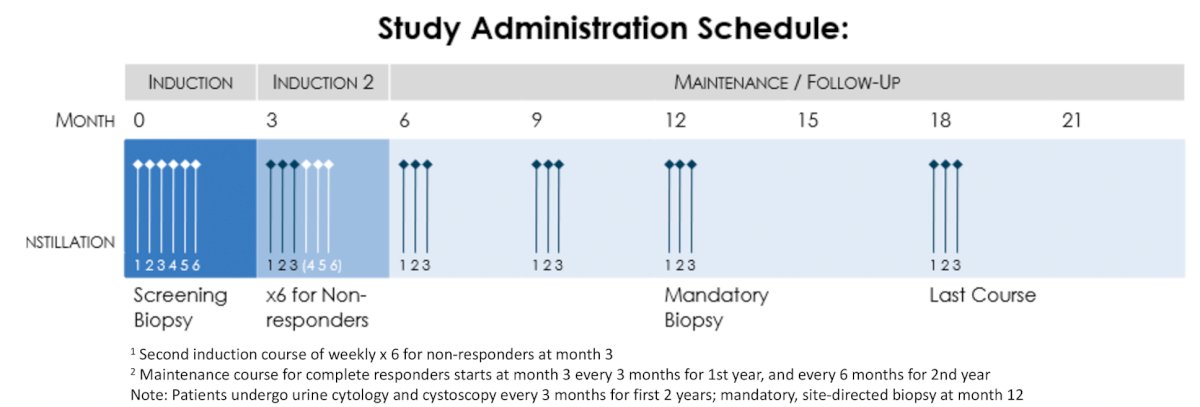
The primary endpoint of the study is complete response at any time on study as assessed by biopsy (directed to cystoscopic abnormalities and mandatory mapping at 12 m), urine cytology, and radiography, as above. Secondary endpoints include complete response at 12 months, duration of response, progression free survival, cystectomy free survival and safety. Correlative assessments include changes in the tumor immune microenvironment, systemic immune induction as reflected in the peripheral blood and urine, as well as viral replication and transgene expression. Baseline expression of coxsackie adenovirus receptor, E2F transcription factor as well as anti-adenovirus antibody titer will be correlated with tumor response. Study enrollment globally is ongoing including in North America, Taiwan, Japan, and South Korea.
Presented By: Edward M. Uchio, MD, University of California, Irvine Medical Center, Orange, CA
Co-Authors: Donald L. Lamm, Neal D. Shore, Ashish M. Kamat, Mark Tyson, Ben Tran, Paul Anderson, Paola Grandi, James M. Burke
Affiliations: BCG Onc, Phoenix, AZ, Carolina Urologic Research Center, Myrtle Beach, SC, The University of Texas MD Anderson Cancer Center, Houston, TX, Mayo Clinic, Scottsdale, AZ, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia, Royal Melbourne Hospital, Melbourne, Australia, CG Oncology, Irvine, CA
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022
References:
- Burke JM, Lamm DL, Meng MV, et al. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol . 2012 Dec;188(6):2391-2397.
- Packiam VT, Lamm Dl, Barocas DA, et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol. Oct;36(10):440-447.


