(UroToday.com) The 2022 GU ASCO Annual meeting included a urothelial carcinoma oral abstract session featuring Dr. Daniel Petrylak and colleagues presenting results of EV-103 Cohort H, assessing antitumor activity of neoadjuvant treatment with enfortumab vedotin monotherapy in patients with muscle-invasive bladder cancer who are cisplatin ineligible. Up to 25% of all patients diagnosed with urothelial cancer present with muscle-invasive disease for whom the risk of progression or metastasis is substantial. Neoadjuvant chemotherapy prior to radical cystectomy and pelvic lymph node dissection has been shown to prolong overall survival for patients who are cisplatin eligible. The standard of care for cisplatin ineligible patients undergoing surgery does not include neoadjuvant therapy. Therefore, safe, and effective neoadjuvant therapies are an unmet need for cisplatin ineligible patients with muscle-invasive bladder cancer. Enfortumab vedotin is an antibody-drug conjugate directed to Nectin-4, which is highly expressed in urothelial cancer, and has been shown to benefit locally advanced or metastatic urothelial cancer patients in Phase II1 and III2 trials, including cisplatin ineligible patients. This study shows preliminary data from Cohort H of the EV-103 phase 1b/2 trial in patients with muscle invasive bladder cancer who are cisplatin-ineligible and treated with neoadjuvant enfortumab vedotin monotherapy.
Cohort H of the EV-103 phase 1b/2 trial (NCT03288545) enrolled patients with cisplatin ineligible cT2-T4aN0M0 muscle-invasive bladder cancer who were eligible for radical cystectomy and pelvic lymph node dissection and had an ECOG of 0-2. Patients received 3 cycles of neoadjuvant enfortumab vedotin (1.25 mg/kg) on Days 1 and 8 of every 3-week cycle prior to radical cystectomy and pelvic lymph node dissection. The primary endpoint of the study was pathological complete response rate (ypT0N0) by central review. Key secondary endpoints included pathological downstaging rate (yp T0,Tis,Ta,T1,N0) and safety. The trial design for EV-103 cohort H is as follows:
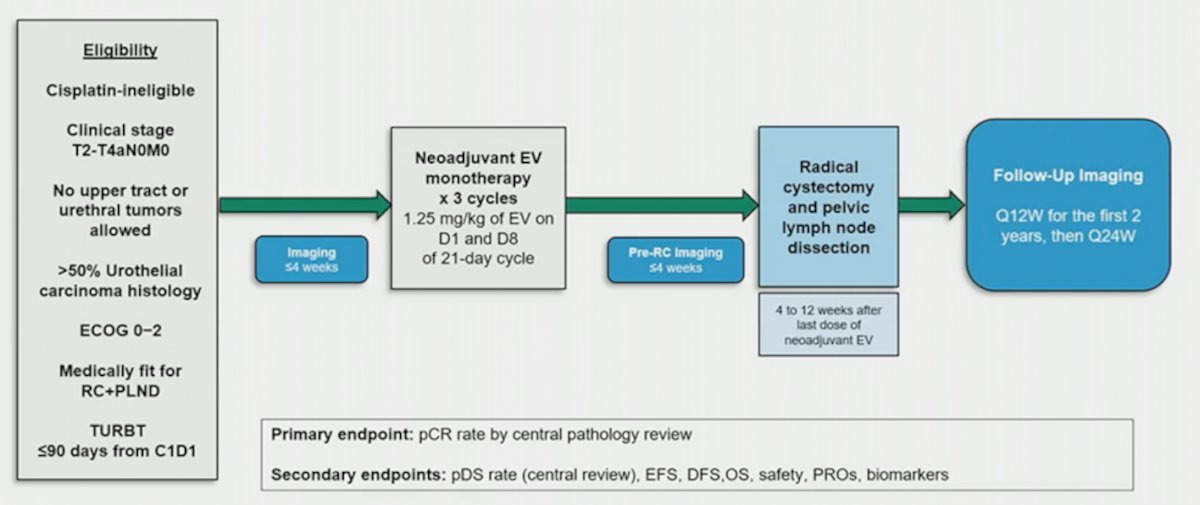
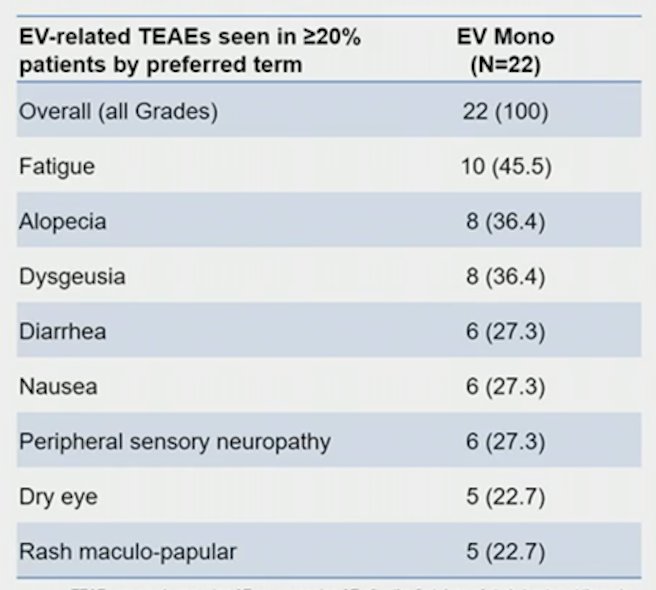
There were 22 patients treated, with the following clinical stage: cT2 (68.2%), cT3 (27.3%), and cT4 (4.5%) tumors. Among these patients, 68.2% patients had predominant urothelial cancer and 31.8% had a mixed histology. The most common reasons for cisplatin ineligibility included creatinine clearance <60 mL/min (50%) and Grade >=2 hearing loss (40.9%). There were 19 patients that completed all three cycles of enfortumab vedotin and 21 underwent radical cystectomy and pelvic lymph node dissection, with one patient having a partial cystectomy. The median time from the end of neoadjuvant enfortumab vedotin to cystectomy was 1.8 months (range: 1.0-2.7). Overall, 36.4% (95% CI 17.2-59.3) of patients had a pathological complete response, and pathological downstaging was seen in 50.0% (95% CI 28.2-71.8) of patients. The most common enfortumab vedotin treatment-related adverse events were fatigue (45.5%), alopecia (36.4%), and dysgeusia (36.4%). There were 18.2% of patients that had Grade ≥3 enfortumab vedotin treatment-related adverse events. No surgeries were delayed due to enfortumab vedotin administration. There were three patients that had Grade 5 adverse events while on study that was unrelated to enfortumab vedotin, and in two patients these adverse events occurred > 30 days after radical cystectomy and pelvic lymph node dissection (one cardiac arrest, one pulmonary embolism). A summary of enfortumab vedotin treatment emergent adverse events seen in >=20% of patients is as follows:
Dr. Petrylak concluded his presentation of EV-103 Cohort H assessing neoadjuvant enfortumab vedotin in cisplatin ineligible patients with muscle invasive bladder cancer with the following take-home messages:
- Observed pathological complete response rate (36%) and pathologic downstaging (50%) after neoadjuvant enfortumab vedotin showed promising activity in cisplatin ineligible patients with muscle-invasive bladder cancer who have a high unmet need
- All patients were able to undergo surgery and there was no delay in surgery due to neoadjuvant enfortumab vedotin
- Adverse events were consistent with the known safety profile of enfortumab vedotin
- This first disclosure of data supports the ongoing Phase II and III programs evaluating enfortumab vedotin in muscle-invasive bladder cancer
Co-Authors: Thomas W. Flaig, Nataliya Mar, Theodore Stewart Gourdin, Sandy Srinivas, Jonathan E. Rosenberg, Maria Guseva, Yao Yu, Sujata Narayanan, Christopher J. Hoimes
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022
References:
- Yu EY, Petrylak DP, O’Donnell PH, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021 May 12;S1470-2045(21)00094-2.
- Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021 Mar 25;384(12):1125-1135.


