(UroToday.com) On the second day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022 focused on urothelial carcinoma, in Poster Session B, Dr. Vignani presented results of the Meet-URO12 trial examining niraparib as maintenance therapy in advanced urothelial carcinoma (UC). Niraparib is an oral inhibitor of poly ADP-ribose polymerase (PARP) enzymes. Platinum-based chemotherapy is the accepted standard of care in first line treatment of advanced UC. Thus, based on the association of mutations in homologous recombination repair (HRR) genes with platinum sensitivity, the authors hypothesized that maintenance treatment with niraparib would improve outcomes among patients who have an objective response (OR) or stable disease (SD) to first line chemotherapy.
In Meet-URO12, a randomized phase II multicentre trial, the authors enrolled patients with advanced transitional cell UC who did not have evidence of progression after 4-6 cycles of first-line platinum-based chemotherapy (cisplatin or carboplatin). Patients were randomized in a 2:1 fashion to to experimental arm A (niraparib 300 or 200 mg daily according to body weight and baseline platelets, plus BSC) or control arm B (BSC alone).
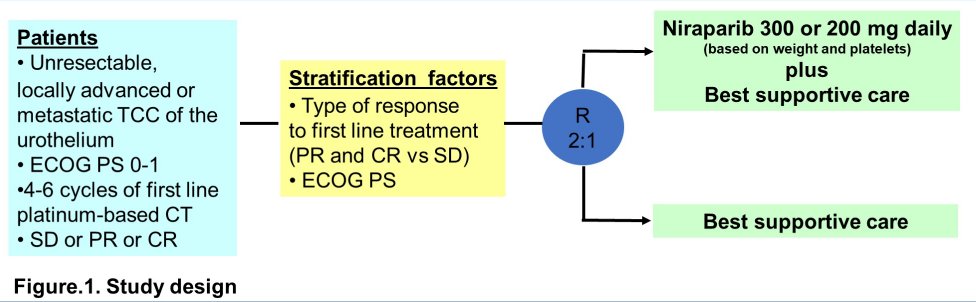
The primary study endpoint was progression-free survival (PFS) and power calculations determined that 77 were required with 65 PFS events were needed to detect hazard ratio of 0.57, with 80% power and one-tailed alpha 0.1. Accrual was prematurely stopped due to approval of avelumab in the same disease indication, and protocol was amended to perform analysis with ≥ 40 PFS events. Molecular characteristics, including alteration of HRR genes, were assessed in formalin-fixed paraffin-embedded tumour samples using the FoundationOne CDx assay.
Between August 2019 and March 2021, 58 patients were randomized in 14 Italian centers of whom 39 were assigned to arm A and 19 to arm B. One of the patients assigned to arm A did not start niraparib. The median age of included patients was 69 years (range 44-84) and the majority (65.5%) had ECOG performance status of 0. In terms of response to platinum-based chemotherapy, 55.2% had an objective response while the remaining 44.8% had stable disease.
As of a data cut-off of August 2021, after a median follow-up of 8.5 months, 47 PFS events were recorded. The median PFS was 2.1 months among those receiving niraparib with best supportive care and 2.4 months in those receiving best supportive care alone (HR 0.92; 95%CI 0.49 – 1.75, p=0.81). The 6-month progression-free rate was 28.2% and 26.3%, respectively.

Among 47 patients with molecular information available, 21 (44.7%) had HRR alterations: 6 (12.8%) known pathogenic mutations and 15 (31.9%) variants of unknown significance. PFS was not longer in patients with either known pathogenic mutations (2.0 vs 1.9 months) or any HRR mutation (2.0 vs 2.0 months) with niraparib.
Any grade≥3 treatment-emergent adverse event (AE) was reported in 25 patients (65.8%) in arm A and in 3 (15.8%) patients in arm B. Further, nearly half (47.4%) of patients required dose reduction of niraparib. The most common AEs associated with niraparib were anemia (50.0%, G3 10.5%), thrombocytopenia (36.8%, G3-4 15.8%), neutropenia (21.1%, G3 5.3%), fatigue (31.6%, G3 15.8%), constipation (31.6%, G3 2.6%), mucositis (13.2%, G3 2.6%), nausea (13.2%, G3 2.6%).
The authors therefore conclude that maintenance niraparib did not improve progression free survival following initial platinum based chemotherapy, a setting in which avelumab has shown survival benefits.
Presented by: Francesca Vignani, MD, Medical Oncology, Ordine Mauriziano Hospital, Turin, Italy

