Despite these advances, there remains a dearth of data for biomarkers of prognosis and response to treatment. In a poster presentation at the 2021 ASCO GU Cancers Symposium, Dr. Johann De Bono and colleagues presented their work assessing the role of circulating tumor cell (CTC) counts as a biomarker of prognosis and response to therapy, among patients enrolled to the CARD trial.
To do so, the authors utilized blood samples collected at the time of screening, on cycle 2 day 1, and at the end of therapy. These samples then underwent analysis for CTC analysis. The authors pre-specified CTC to be any cytokeratin-positive, CD45-negative cells.
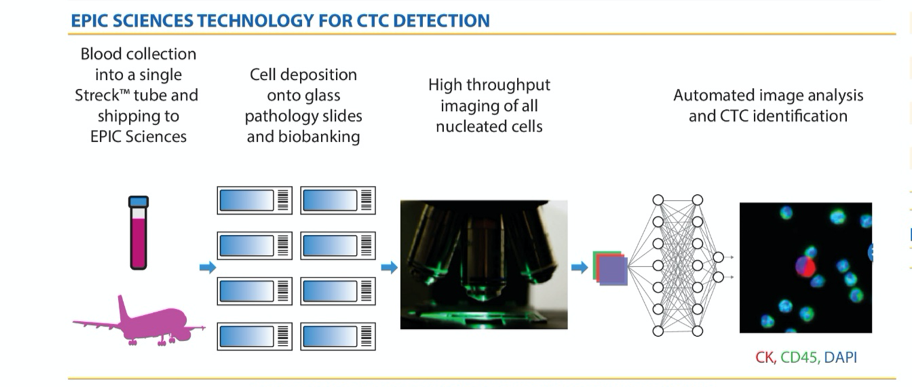
The authors then tested the association between CTC counts and time-to-event endpoints (rPFS and OS) and categorical outcomes (objective tumor response) using multivariable Cox models and chi-squared tests, respectively.
Among 255 patients randomized within the CARD trial, 237 (93%) had evaluable samples at screening and 213 (84%) had evaluable samples on day 1 of cycle 2. At least one CTC were detected in 204 samples (86%) at screening at 178 samples (84%) at cycle 2 with the median number of CTCs of 4.1 (interquartile range 1.3-14.2) and 5.2 (IQR 1.5-17.3) at each time point respectively.
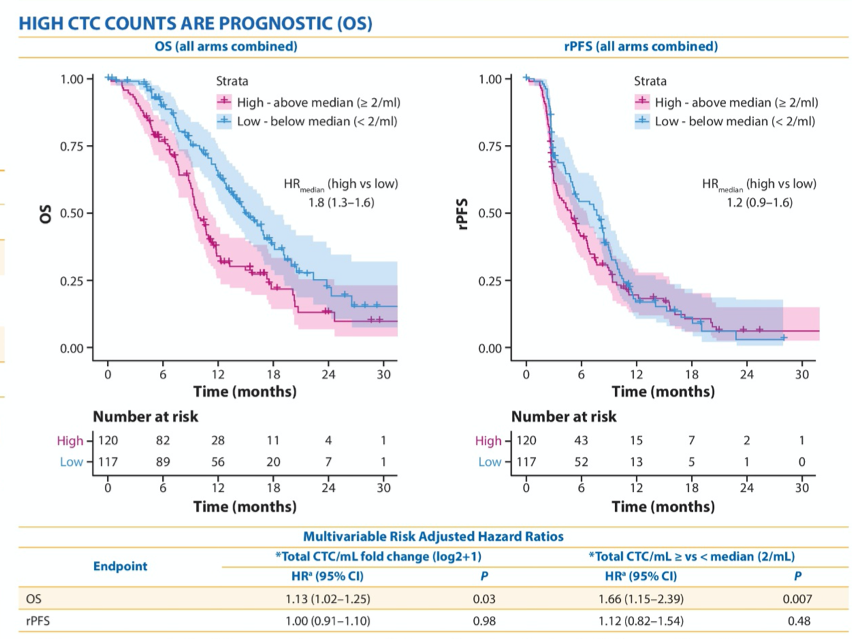
From baseline assessment performed at the time of screening, higher CTC counts were associated with other poor prognostic factors including higher levels of lactate dehydrogenase (p=0.014), alkaline phosphatase (p<0.001), and prostate specific antigen (p<0.001). Further, measured continuously, higher CTC counts were independent with poorer overall survival (p=0.03) but not differences in rPFS (p=0.7). Similarly, when dichotomized around the median, there was improved OS for patients with low CTC counts but no difference in rPFS.
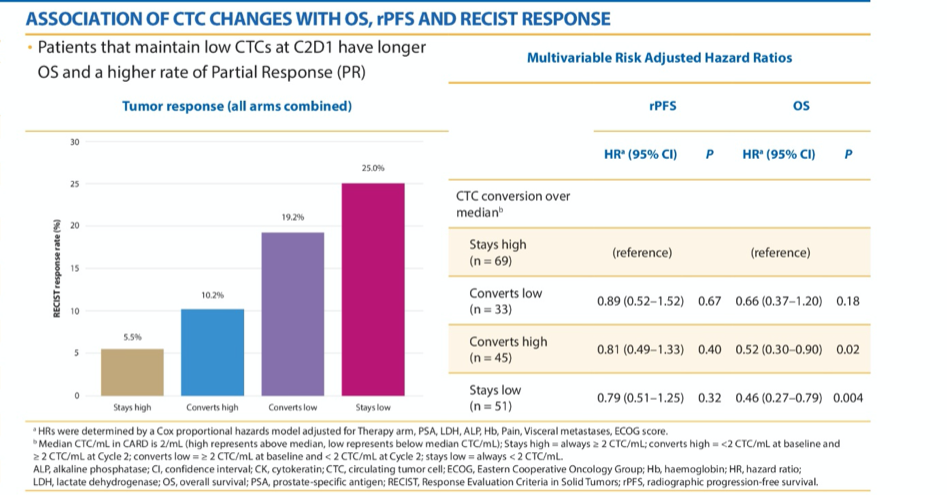
Considering next the change in absolute CTC count from the screening visit to cycle 2, the authors found that (including patients in both treatment arms), there was a benefit in terms of pRFS for patients who had favourable changes in CTC count. Additionally, conversion from high to low or maintenance of low CTC counts was associated with objective tumor response.
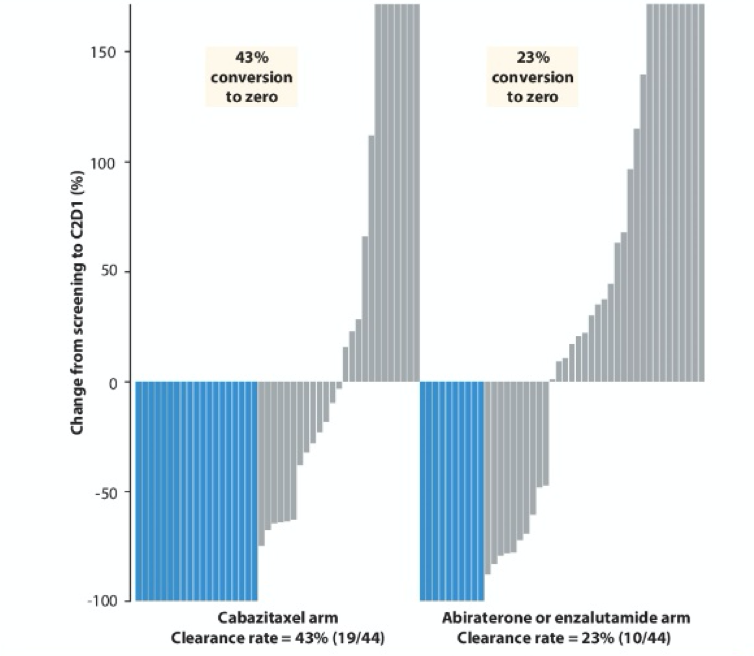
Further, clearance of high-CK expressing cells was associated with improved rPFS (p=0.006) and OS (p=0.005). This was more commonly observed among patients who received cabazitaxel.
In summary, the authors conclude that this pre-planned analysis of the CARD trial demonstrates that baseline CTC counts are prognostic among patients undergoing third-line systemic therapy for mCRPC. Further, early favourable changes in CTC status (ie. conversion to undetectable) are associated with the response to therapy. Ongoing work will assess whether CTC changes may be used as a surrogate for survival, thus allowing early outcome ascertainment. Further, analysis of the associations of CTC subtypes including androgen receptor variant 7, chromosomal instability, heterogeneity, small-cell/neuroendocrine morphology is ongoing.
Presented by: Johann de Bono, MB ChB FRCP MSc Ph.D. FMedSci. Professor Johann de Bono is Regius Professor of Cancer Research and a Professor in Experimental Cancer Medicine at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust.
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021
Related Content:
Clinical Trial Information: NCT02485691


