Multiple diagnostic and therapeutic technologies focused on prostate-specific membrane antigen (PSMA) are becoming available. PSMA is a type II transmembrane glycoprotein that is expressed in the cytoplasm on the apical side of prostate epithelium surrounding prostate ducts. In prostate cancer, PSMA is significantly overexpressed on the vast majority of prostate cancer cells and is expressed on the luminal surface of prostate ducts when prostate cancer develops. A strength of PSMA-coupled imaging is improved diagnostic accuracy even at PSA levels of 0.5 or less, as shown by studies including proPSMA,1 which was conducted in high-risk localized prostate cancer where the risk of dissemination not detected on conventional imaging is higher. PSMA-based imaging has the potential to change clinical management options in a significant percentage of cases, though the long-term impact of earlier detection on patient outcomes remains to be determined. Additionally, PSMA expression can be quite heterogeneous, and advanced tumors lacking PSMA expression but having high FDG uptake on conventional PET represent an especially poor-risk subset of tumors.
Coupling the lutetium-177 (beta emitter) or actinium-225 (alpha emitter) with PSMA to generate PSMA radionuclide therapeutics allows for the therapeutic targeting of PSMA-expressing tumors. A schematic of how these technology works are shown below, whereby radiopeptide only is internalized in cells that express PSMA on their surface, resulting in DNA damage breaks and tumor cell death.

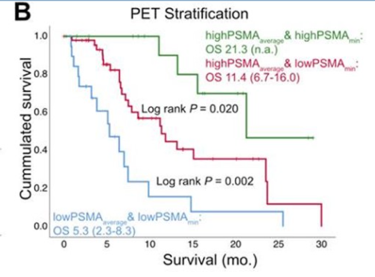
Who could receive PSMA-radionuclide therapy? Patients require adequate renal function without any urinary tract obstruction and limited cytopenias. Tumors must be found to express PSMA in order to be eligible for therapy, and so far the studies have been for management in the advanced setting, especially post-taxane (TheraP). Patients whose tumors express higher levels of PSMA by PET scan tend to respond better and have longer overall survival relative to patients with tumors expressing low levels of PSMA.
Dr. Sathekge then previously summarized recent results from the TheraP trial, comparing lutetium-177 PSMA therapy against cabazitaxel in castration resistant disease, which demonstrated higher rates of PSA response and fewer severe side effects for PSMA radionuclide therapy.

He then highlighted two especially interesting clinical questions surrounding PSMA radionuclide therapy: (1) will patients be able to get multiple PSMA-targeted therapies such as actinium and lutetium, and (2) can you re-challenge patients who previously received lutetium with lutetium a second time? The first question remains to be studied but is important given preliminary data of the efficacy of actinium in advanced disease. For the second question, he cited a recent study2 where 15 patients who had previously received lutetium-177-PSMA were rechallenged and 11 patients experienced a PSA decline greater than 50%, whereas only 4 of 21 patients who went on to receive other systemic therapies had a similar degree of PSA response. The median duration of response with lutetium rechallenge was less durable than initial response.
Dr. Sathekge next discussed an important side effect of PSMA radionuclide therapeutics, xerostomia, which occurs because of PSMA expression in the salivary glands. Options for avoiding this toxicity include dose de-escalation or a new class of albumin-binding PSMA radioligands with less salivary gland uptake.
He concluded his talk by discussing future directions in radionuclide therapy, including possibly combining androgen-pathway inhibition with lutetium-PSMA, and other novel radionuclides such as I123-PARP, which may specifically target tumors with high PARP expression.
Presented by: Mike Sathekge, MBChB, MMed (Nucl Med), PhD, University of Pretoria and Steve Biko Academic Hospital
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021
References:
- Hofman M, Lawrentschuk N, Francis R et al. "Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study." The Lancet. 2020. 395, ISSUE 10231, P1208-1216.
- Violet J, Sandhu S, Iravani A et al. "Long-Term Follow-up and Outcomes of Retreatment in an Expanded 50-Patient Single-Center Phase II Prospective Trial of 177Lu-PSMA-617 Theranostics in Metastatic Castration-Resistant Prostate Cancer." The Journal of Nuclear Medicine. 2020. 61 (6) 857-865.


