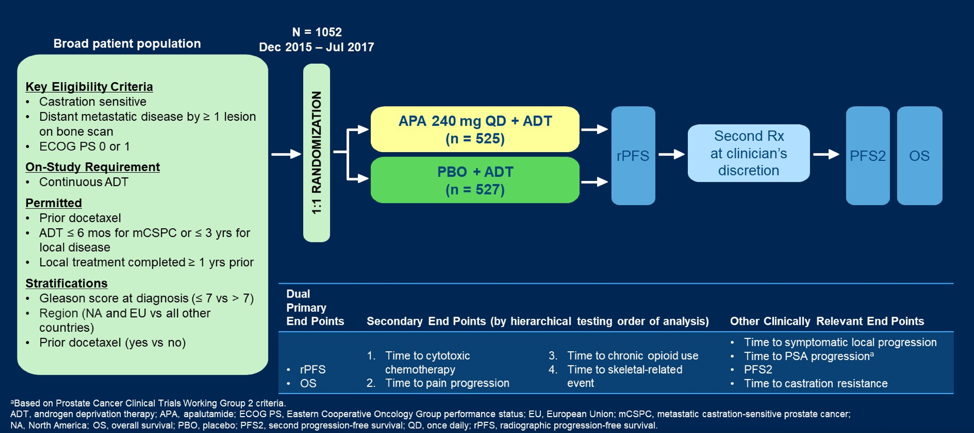
In TITAN, 1,052 mCSPC patients were randomized 1:1 to receive apalutamide (240 mg QD) or placebo plus ADT. Time-to-event end points were analyzed by Kaplan-Meier method and Cox proportional hazards model. A preplanned sensitivity analysis for overall survival, accounting for crossover using inverse probability censoring weighted (IPCW) log-rank test, was conducted. No formal statistical retesting was performed; nominal p values were reported without multiplicity adjustment. Change from baseline in Functional Assessment of Cancer Therapy-Prostate (FACT-P) total score was assessed using a mixed-effect repeated-measures model. As follows is the trial design for TITAN:
With 44 months median follow-up, there were 405 overall survival events that occurred. After unblinding, there were 208 placebo patients (39.5%) who crossed over to apalutamide. Median treatment duration was 39.3 months for the apalutamide group, 20.2 months for the entire placebo group, and 15.4 months for the patients that crossed over from placebo to apalutamide crossover group. In the updated analysis apalutamide plus ADT reduced the risk of death by 35% (HR 0.65, 95% CI 0.53-0.79):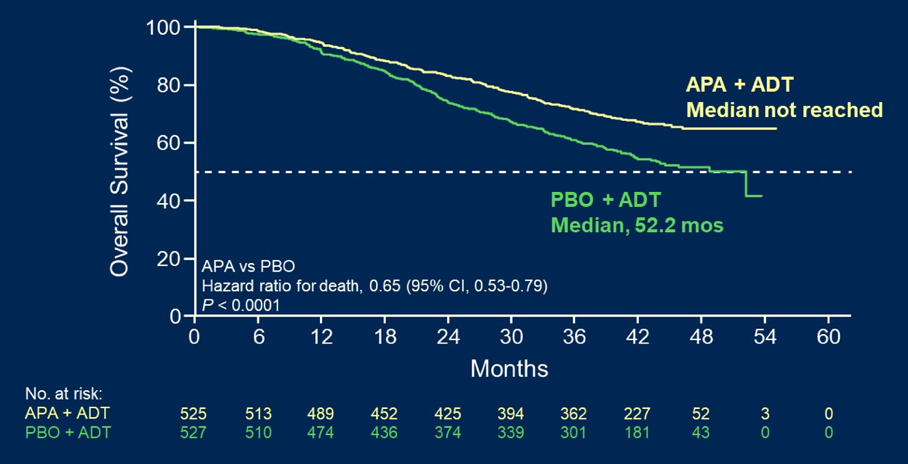
Furthermore, after adjusting for treatment crossover, apalutamide plus ADT had a 48% reduction in risk of death (HR 0.52, 95% CI 0.42-0.64):
The 48-month survival rates were 65% for apalutamide compared to 52% for the placebo group. Other endpoints also favored apalutamide versus placebo, including second progression-free survival (HR 0.62, 95% CI 0.51-0.75) and time to castration resistance (HR 0.34, 95% CI 0.29-0.41). Health-related quality of life, per total FACT-P, was maintained in the apalutamide group through the study and was not different from the placebo group. Additionally, safety was consistent with previous reports.
Dr. Chi concluded his presentation of the final analysis of TITAN with the following take home messages:
- With close to 4 years of follow-up, the final analysis of TITAN demonstrated that in a broad population of patients with mCSPC, apalutamide plus ADT provides an improvement in overall with a 35% reduction in risk of death, which increased to 48% reduction after adjusting for patients who crossed over from placebo to apalutamide
- In addition, there was consistent benefit with apalutamide in other end points, including delaying castration resistance, and health-related quality of life continued to be maintained with an acceptable safety profile
Presented by: Kim N. Chi, MD, Senior Research Scientist, Vancouver Prostate Centre, Chief Medical Officer & Vice President, BC Cancer, Medical Oncologist, BC Cancer – Vancouver, Professor, Department of Medicine, UBC, Vancouver, BC, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021References:


