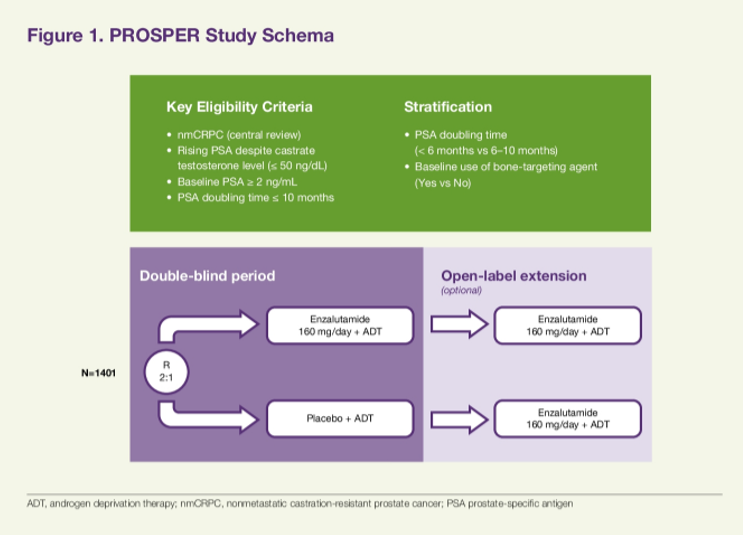
The methodology of the PROSPER trial has previously been reported and published. In brief, men with nmCRPC, PSA doubling time ≤ 10 months, and PSA ≥ 2 ng/mL at screening were randomized in a 2:1 fashion to enzalutamide 160mg or placebo, in addition to continuing androgen deprivation therapy.
Given the primary outcome, as previously reported, of metastasis-free survival, assessment of overall survival was assessed as a key secondary outcome utilizing a group sequential testing procedure with O’Brien-Fleming-type alpha spending function. In this analysis, the authors performed a multivariable analysis assessing both OS and exposure-adjusted adverse events (AEs), including age (≤ 70 yrs and > 70 yrs), geographic region, and other variables.
In this post-hoc analysis, the authors demonstrated that the overall survival benefit associated with the use of enzalutamide in nmCRPC was consistent across age groups and geographic regions. Compared to placebo, enzalutamide reduced the risk of death similarly in patients aged ≥ 70 yrs (hazard ratio [HR] 0.73; 95% CI 0.58-0.9) and those aged < 70 yrs (HR 0.72, 95% CI 0.5-1.04).
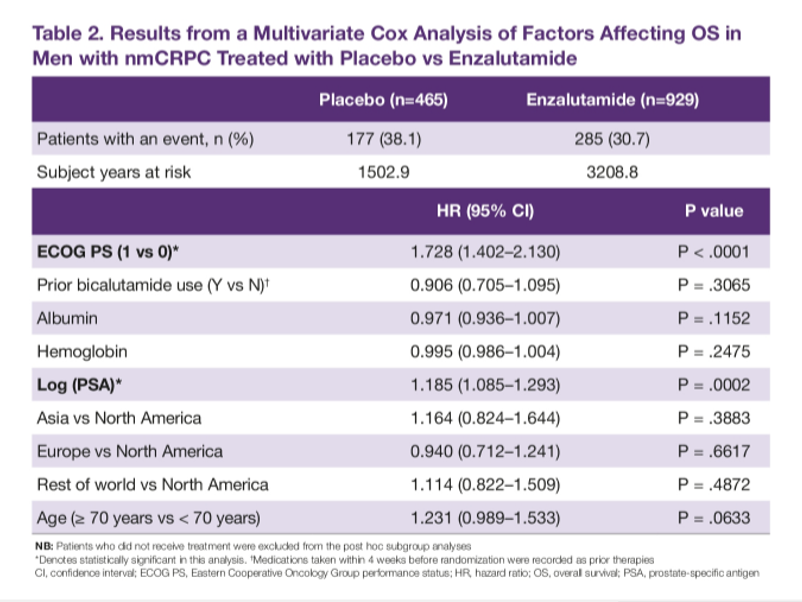
While age and geographic region didn’t appear to significantly affect outcomes, the authors identified 3 factors associated with mortality: Eastern Cooperative Oncology Group (ECOG) performance status (1 vs 0; HR 1.7; 95% CI 1.4-2.1), a log of PSA (HR 1.2; 95% CI 1.1-1.3), and use of subsequent therapy (yes vs no; HR 2.5; 95% CI 2.1-3.1).
As with efficacy outcomes, overall safety did not differ between age groups and across geographic regions.
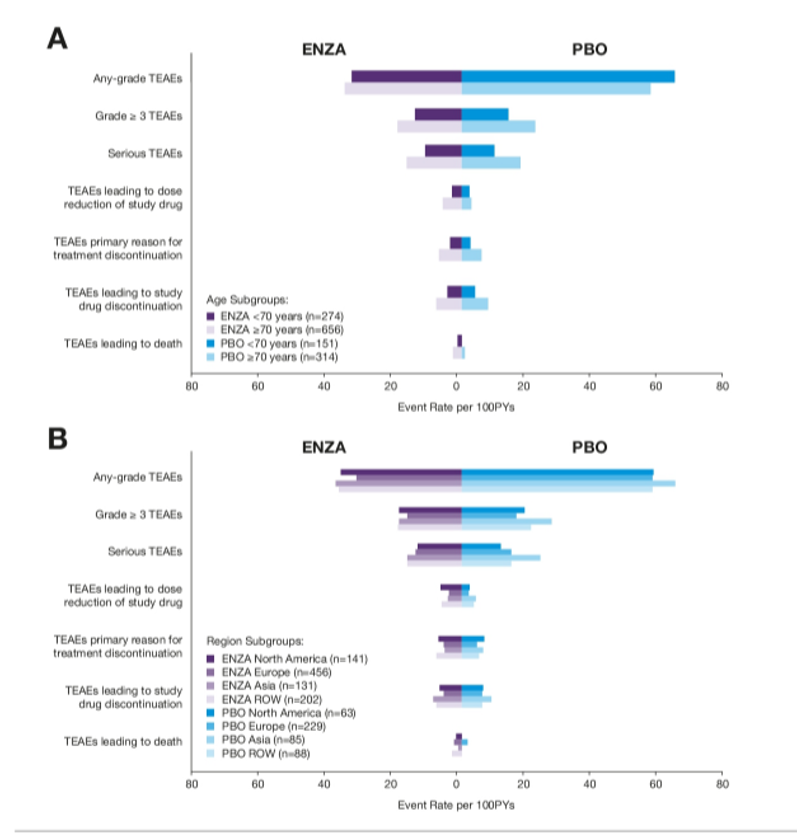
The authors, therefore, conclude that the benefit and toxicity of enzalutamide in nmCRPC are similar regardless of age and geographic region.
Presented by: Ugo De Giorgi, Italian Ministry of Health, Scientific Institute of Romagna for the study and treatment of tumors, in Bologna, Italy
Co-Authors: Maha H. A. Hussain, Neal D. Shore, Karim Fizazi, Bertrand Tombal, David F. Penson, Fred Saad, Eleni Efstathiou, Katarzyna Madziarska, Joyce Leta Steinberg, Jennifer Sugg, Xun Lin, Qi Shen, Cora N. Sternberg
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Twitter @WallisCJD during the 2021 ASCO Genitourinary Cancers Symposium (ASCO GU), February 11th to 13th, 2021


