(UroToday.com) In plenary presentation in the Progress and Promise in Treatment Personalization for Advanced Prostate Cancer session at the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU), Dr. Karen Autio discussed ways in which we can harness the immune system to treat prostate cancer, emphasizing the role of immune checkpoint inhibitors in prostate cancer.
To begin, she discussed approved indications for use of immune checkpoint monotherapy in prostate cancer. In unselected patients, she emphasized that there is no proven benefit. However, there are two tumor agnostic approvals for anti-PD1 therapy that are relevant to prostate cancer: first, among patients with mismatch repair deficiency and second among patients with high tumor mutational burden. However, despite relevant approvals, relatively few patients with prostate cancer were enrolled in these trials.
She, therefore, concluded that testing for MMR and TMB is relevant in appropriate patients.
In the context of immunotherapy, Dr. Autio highlighted a number of potentially relevant characteristics of prostate cancer include the presence of bone predominant disease and bone microenvironment, the use of androgen deprivation therapy, and the ability to use PSMA for targeting.
She emphasized the well-described “vicious cycle of bone metastases” with growth factors and pro-inflammatory cytokines present in the pre-metastatic setting. Chemokines then assist with the dissemination of tumor cells to bone. These tumor cells secrete IL-6 and other factors leading to the activation of osteoblasts which in turn secrete RANKL. This secreted RANKL then binds with RANK on osteoclasts leading to a release of growth factors that feedback and continue this cycle. While this cycle has obvious effects on bone remodeling, the growth factors and cytokines involve also effect dendritic cells, lymphocyte development, and T cell response.
An observation that patients without bone metastases may fair better with use of checkpoint inhibitors led to work demonstrating that osseous involvement leads to the release of TGF-beta and IL-6 which is absent in those without bony involvement. The use of anti-CTLA-4 agents was associated with induction of a (Th)17 lineage but not Th1 effector cells in bony metastases. However, inhibition of TFG-beta allowed Th1 development and subsequent tumor response to checkpoint inhibitor therapy.
In the bone tumor microenvironment, there are a number of relevant immune suppressive cells including myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), and regulatory T cells (Treg). The presence of these cells is associated with disease progression and prognosis. In particular, pre-clinical models suggest that bone-specific macrophages may affect the response. Interestingly, PTEN deficient tumors (as discussed in Dr. Gleave’s talk in the same session) are associated with a higher density of Treg cells in both the stroma and tumor. Such an effect is also seen in other tumor types, including melanoma where it is associated with response to checkpoint inhibition.
Moving to discuss the role of ADT, Dr. Autio emphasized that ADT induce an immune infiltrate within tumors that involves CD8 T-cells, CD56 macrophages, and Treg cells. This may have implications for tumor growth in the peri-castration setting, that dissipates in the castration-resistant space, at least in pre-clinical models. Further, ADT shows evidence of remodeling of the tumor microenvironment in the hormone-sensitive space, with upregulation of immune-related genes and an increase in T cell infiltration.
Dr. Autio then discussed the potential for PSMA targeting in immunotherapy. In addition to diagnostic utility, PSMA may be used as a pharmacodynamic measure. Further, there has been considerable interest in the use of PSMA for cancer vaccination though, to date, there have been a significant barrier to implementation of this including self-tolerance, down-regulation of MHC, deficits in antigen-presenting cells, the inability to home to tumor, and a lack of activated T cells.
To overcome immune resistance in prostate cancer, a number of approaches may be undertaken. First, we may attempt to overcome an immunosuppressive pathway that confers resistance by, for example, inhibiting TGF-beta. Second, we may stimulate an underactive process, such as increasing the maturation of dendritic cells or antigen-presenting cells. Third, we may create supra-physiologic environments in order to bypass standard mechanisms for immune activation, including, for example, the use of BiTEs, BIKES/TRIKES, and CARS, approaches that exploit PSMA.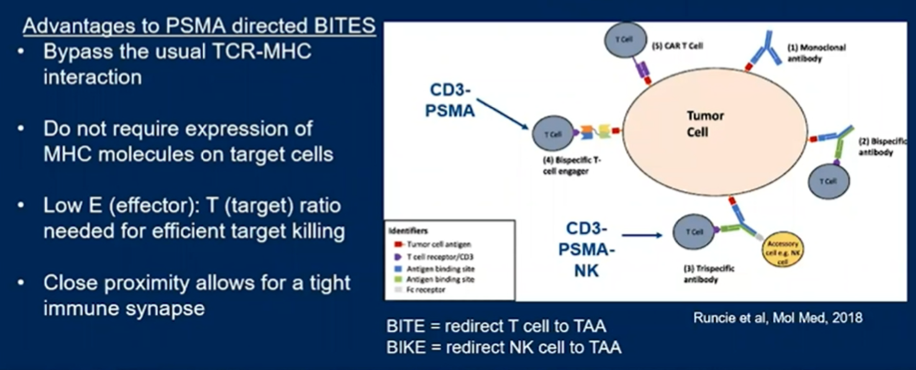
In prostate cancer, a number of BiTEs have been developed. Early data has suggested generally favorable responses among patients with advanced metastatic castration-resistant prostate cancer (mCRPC). They may function via a cytolytic synapse triggering target cell apoptosis, but also by antigen spreading. The predominant toxicity is cytokine release syndrome.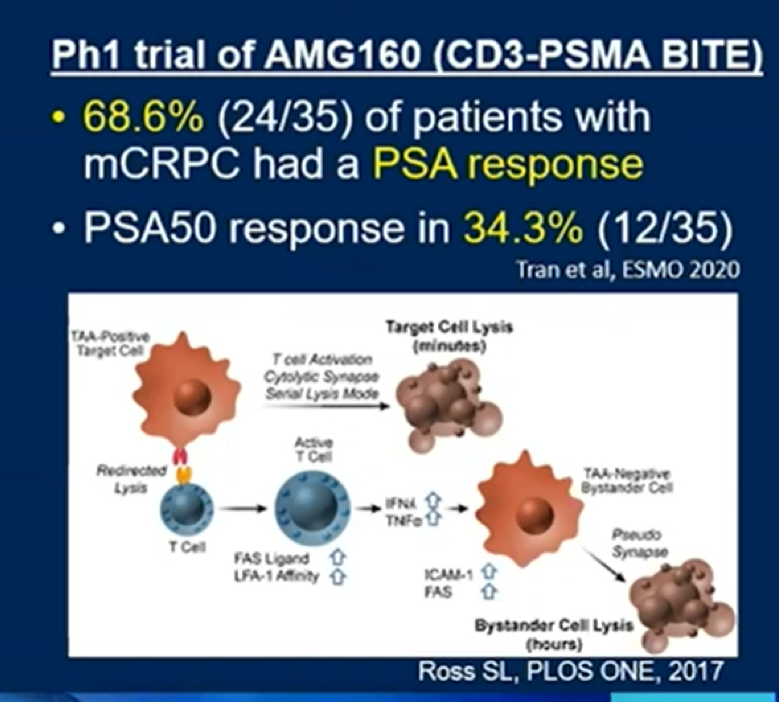
Genetically engineered T cell receptors (CAR-Ts) are an alternative PSMA-based targeted treatment approach.
Dr. Autio concluded that MMR and TMB are, to date, the only approved indications for anti-PD1 agents in mCRPC. However, the nature of prostate cancer, the bone microenvironment, and prostate cancer treatments make it an appealing target for further development of immune targeting treatment approaches.
Presented by: Karen A. Autio, MD, MSc, Medical Oncologist, Memorial Sloan Kettering Cancer Center
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, Contact: @WallisCJD on Twitter during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021


