The Genomics in Michigan ImpactiNg Observation or Radiation (G-MINOR) randomized trial enrolled participants across 12 centers between January 2017-August 2018. Eligible patients had undergone radical prostatectomy within 9 months of enrollment, had pT3-4 disease and/or positive surgical margins, and a PSA < 0.1 ng/mL. Patients were assigned to either the genomic classifier or usual care group using cluster-crossover block randomization. Patients and providers in both arms received a CAPRA-S recurrence risk score. Decipher scores were obtained on radical prostatectomy tissue of all patients, but patients and providers in the usual care arm were blinded to the results. The primary endpoint was the impact of the genomic classifier test result on adjuvant treatment decisions compared to clinical factors alone within 18 months of radical prostatectomy. The trial design for the G-MINOR trial is as follows:

There were 356 patients randomized and 340 had at least 18 months of follow-up. Randomization resulted in 175 (51.5%) genomic classifier patients and 165 (48.5%) usual care patients. There were no significant differences in clinical variables or Decipher scores between the arms. At 18 months post-radical prostatectomy, 19 (10.9%) patients in the genomic classifier group and 12 (7.3%) patients in the usual care group had received adjuvant treatment. In the primary analysis, availability of the genomic classifier score in the genomic classifier arm was significantly associated with adjuvant treatment in genomic classifier high-risk patients after controlling for CAPRA-S risk (OR 7.6, 95%CI 1.95-29.6):
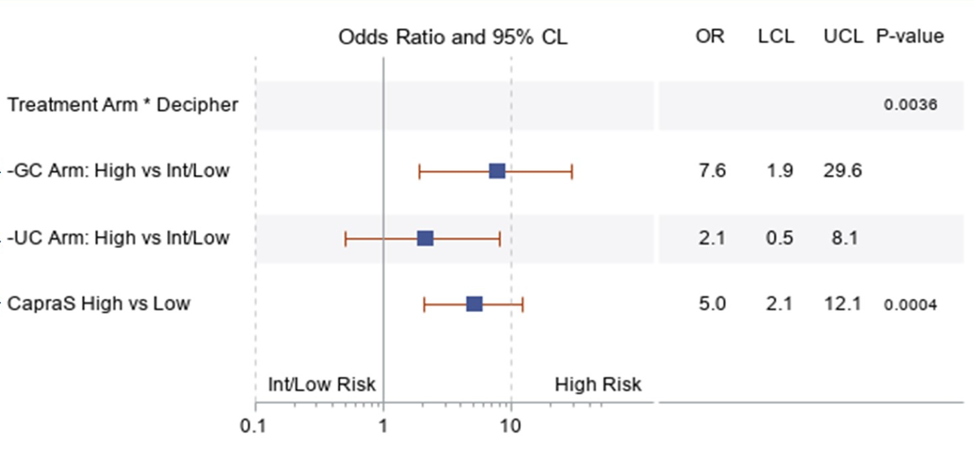
In the genomic classifier arm, both genomic classifier score (OR 8.8, 95%CI 1.9-39.7) and CAPRA-S score (OR 3.8, 95%CI 1.09-12.9) were independently associated with adjuvant treatment in a multivariable logistic regression model.
Dr. Morgan concluded his presentation of the G-MINOR trial with the following concluding remarks:
- This was the first prospective, randomized assessment of molecular classifier utility in prostate cancer
- The primary analysis demonstrated that the Decipher genomic classifier impacts adjuvant radiotherapy use following radical prostatectomy
- Prospective serial patient reported outcome collection (MUSIC-PRO) will provide key quality of life data
- Long-term oncologic outcome assessment will inform clinical impact of Decipher in this context
Presented by: Todd M. Morgan, MD, Michigan Urological Surgery Improvement Collaborative; University of Michigan, Ann Arbor, MI
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md during the 2021 ASCO Genitourinary Cancers Symposium (ASCO GU), February 11th to 13th, 2021
References:
Related Content:
Validation of a 22-Gene Genomic Classifier in Patients With Recurrent Prostate Cancer An Ancillary Study of the NRG/RTOG 9601 Randomized Clinical Trial


