(UroToday.com) The field of advanced prostate cancer has rapidly progressed over the past 15 years. In the past 5 years, there has been considerable development in the treatment of patients with metastatic castration-sensitive prostate cancer (mCSPC), including chemotherapy and novel hormonal therapies (NHT). Each of these has proven survival benefits compared to the historic standard of androgen deprivation therapy (ADT) alone. However, the uptake of these guideline-recommended treatment approaches with level 1 data is unknown. In a plenary abstract presentation in the Poster Highlights Session: Prostate Cancer session at the 2021 ASCO GU Cancers Symposium, Dr. Stephen Freedland and colleagues assessed utilization and outcomes for patients with mCSPC in the Veterans Health Administration system.
The authors used the Veterans Health Administration (VHA) claims data to identify patients with mCSPC who initiated ADT alone or ADT with anti-androgen, docetaxel, or abiraterone from April 1, 2014, to March 31, 2018. The authors examined the time to metastatic castration-resistant prostate cancer (mCRPC) and overall survival (OS) between these groups using propensity scores calculated using logistic regression accounting for demographics (age, race) and patient characteristics at index (index year to ADT, radiation therapy, chronic corticosteroid use, Charlson Comorbidity Index score, select comorbidities, log(PSA), hemoglobin, alkaline phosphate, site of metastasis). The subsequently used Cox proportional hazard (CPH) models to evaluate time to progression to mCRPC and OS using stabilized inverse probability of treatment weighting (SIPTW) adjusting for body mass index, site of metastasis, time from metastatic diagnosis to index date, and log(PSA).
The authors identified 1395 men with mCSPC, of whom 874 (63%) received ADT only, 338 (24%) received ADT + anti-androgen (98% with bicalutamide), 75 (5%) received ADT + abiraterone, and 108 (8%) received ADT + docetaxel.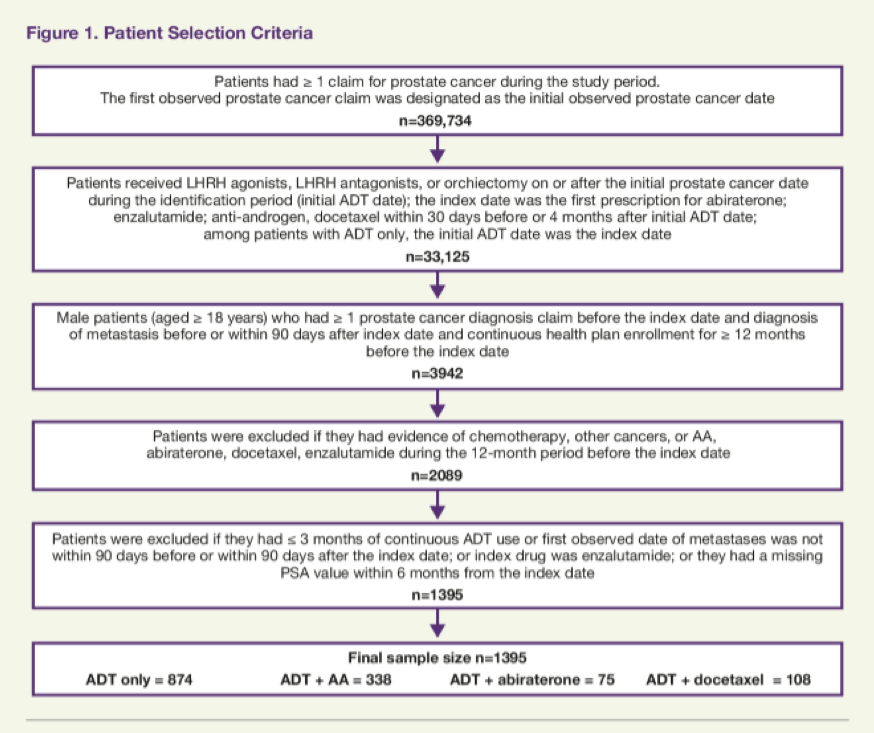
The low utilization of abiraterone and docetaxel precluded further assessment of survival outcomes in these groups.
However, comparing patients who received ADT alone to those receiving ADT + anti-androgen demonstrated a similar risk of progression to mCRPC (hazard ratio [HR]: 1.05 (95% CI: 0.87–1.26, P = .622), with a median time to mCRPC of 21.74 in patients receiving ADT + anti-androgen vs 22.5 months in those receiving ADT only.
Patients treated with ADT+AA had a similar risk of death compared to ADT only (HR: 1.22; 95% CI: 0.97–1.54, P = .093), with median survival not reached in both cohorts.
These data highlight that most patients with mCSPC in the VHA who initiated treatment between 2014 and 2018 were treated with ADT only, despite level 1 evidence supporting the use of docetaxel and NHT strategies.
Presented by: Stephen J. Freedland, MD, Division of Urology, Department of Surgery, Cedars-Sinai Medical Center
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter during the 2021 ASCO Genitourinary Cancers Symposium (ASCO GU), February 11th to 13th, 2021


