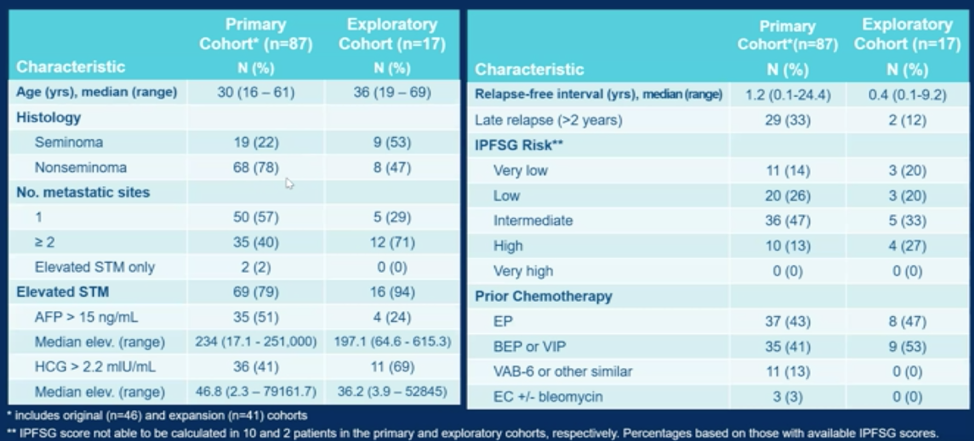
As shown below, there are a few notable differences between the primary cohort and the experimental cohort. The primary cohort had a younger median age of 30, had more non-seminoma patients, and the majority of patients had only 1 metastatic site at the time of salvage therapy. The median relapse-free interval for patients in the primary cohort prior to requiring salvage was 1.2 years. Patients in both cohorts fit into all but the very high IPFSG risk grouping despite qualifying as MSKCC favorable risk.
The favorable response rate, defined as the number of patients attaining either a complete response or partial response with negative tumor markers, was 80% in the primary cohort and 76% in the exploratory cohort. The 5-year progression free median survival was 70% and 56% in each cohort, respectively. This translated into a 5 year median overall survival of 72% for the primary cohort and 59% for the exploratory cohort.
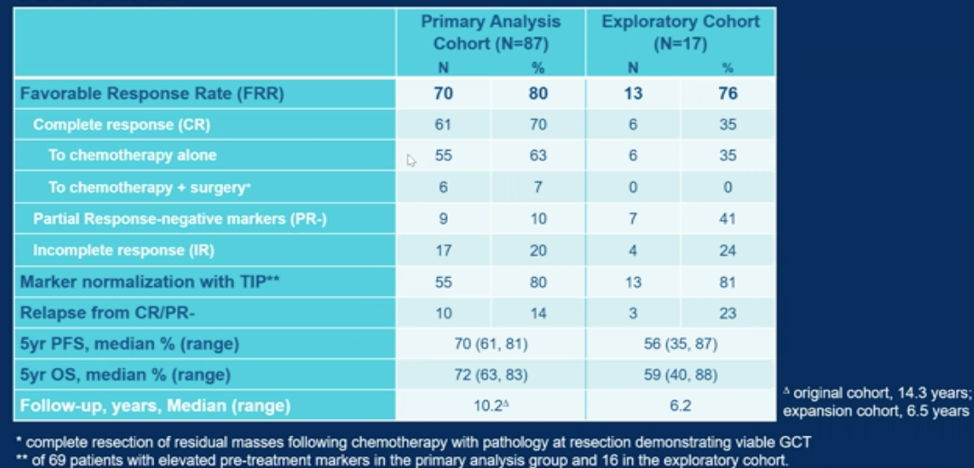
Dr. Gleeson concluded that these data continue to support the use of TIP chemotherapy for the management of patients with relapsed germ cell tumors and MSKCC favorable risk criteria. Patients in an exploratory cohort that did not meet MSKCC favorable risk criteria also had better than expected outcomes with TIP therapy. To further delineate optimal initial salvage therapy in germ cell tumors, the TIGER phase 3 trial is ongoing, randomizing patients to TIP versus TI-CE.
Presented by: Jack Gleeson, MD, Memorial Sloan Kettering Cancer Center
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021
References:
- Kondagunta G, Bacik J, Donadio A et al. "Combination of Paclitaxel, Ifosfamide, and Cisplatin Is an Effective Second-Line Therapy for Patients With Relapsed Testicular Germ Cell Tumors." Journal of Clinical Oncology. 2021. 10.1200/JCO.2005.19.638


