(UroToday.com) Following presentations by Dr. Robert Motzer discussing results of the CLEAR study of lenvatinib plus pembrolizumab or everolimus versus sunitinib in first-line treatment of advanced renal cell carcinoma and by Dr. Sumanta Pal looking at the SWOG 1500 trial of sunitinib, cabozantinib, crizotinib, and savolitinib in patients with metastatic papillary renal cell carcinoma, Dr. Stephanie Berg provided a discussion of these data in the Oral Abstract Session: Renal Cell Cancer session at the 2021 ASCO GU Cancers Symposium.
Dr. Berg first discussed the CLEAR study, beginning by emphasizing the many changes that have happened in the treatment landscape between 2005 and today. Recently, the combinations of immune checkpoint inhibitors with anti-angiogenic agents have a beneficial effect due to their differing mechanisms of action.
She highlighted that CLEAR is a 3-arm, phase III trial examining first-line lenvatinib and everolimus or lenvatinib and pembrolizumab versus sunitinib. She emphasized that the use of a non-IO control arm is relatively unique.
Examining progression-free survival, Dr. Berg highlighted that the curves begin to diverge early, as soon as 2 months following index. The combination of lenvatinib and pembrolizumab maintained superiority throughout the study period. Assessing overall survival, we again see a relatively early separation. Notably, per protocol, those in the lenvatinib and pembrolizumab arm ceased pembrolizumab at 24 months. Dr. Berg noted that the sunitinib arm crosses over towards the end of the curve, though there are relatively few events at these time points.
Dr. Berg then emphasized that treatment-related adverse events were common and more common in the combination arms. However, the only immune-related adverse event examined (hypothyroidism) was somewhat higher among patients receiving pembrolizumab.
Dr. Berg then concluded that CLEAR clearly demonstrates the efficacy of lenvatinib and pembrolizumab with an impressive ORR of 71%. While lenvatinib and everolimus also had superior ORR and PFS compared to sunitinib, only lenvatinib and pembrolizumab demonstrated improvements in overall survival. Further, toxicity was manageable with treatment discontinuation in approximately 10% of patients receiving lenvatinib and pembrolizumab. Thus, lenvatinib and pembrolizumab is another novel combination in this disease space, joining three existing combination approaches of IO/TKIs.
Dr. Berg noted that direct head-to-head data are unlikely to be forthcoming. However, she also emphasized that the use of IO/TKI combinations in the first-line setting makes choice of second line agents less clear.
She then discussed SWOG 1500, as presented by Dr. Pal. She emphasized that papillary RCC is the most common non-clear cell subtype of the disease. To date, sunitinib has been the standard of care for metastatic pRCC, though PFS ranged from 2-8 months. Further, pRCC have distinct oncogenic drivers, including MET. The SWOG 1500 trial randomized patients in a 1:1:1:1 fashion to receive either sunitinib 50 mg oral daily (6-week cycles: 4 weeks on/2 weeks off), cabozantinib 60 mg oral daily, crizotinib 250 mg orally twice daily, or savolitinib 600 mg oral daily. Notably, these last two arms were closed for futility. Dr. Berg noted that while type II pRCC was predominant, differences were smaller at central review.
As reported by Dr. Pal, cabozantinib demonstrated superior progression-free survival compared to sunitinib in this patient population with manageable toxicity. As a result, cabozantinib is another first-line treatment option for patients with metastatic pRCC. Dr. Beg emphasized the comparison between SWOG 1500 and SAVOIR.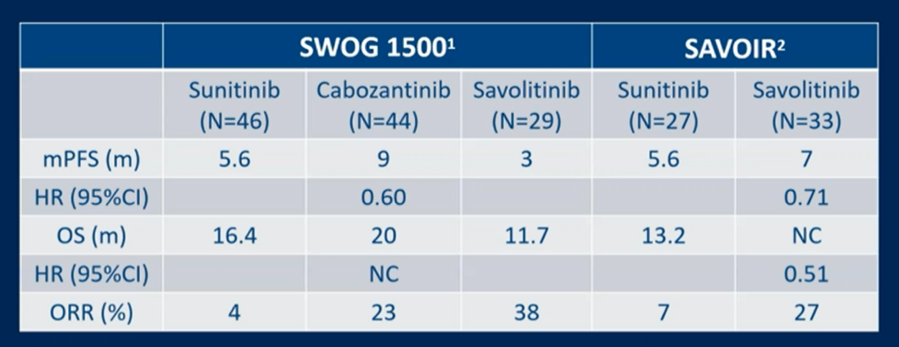
SAVOIR was terminated early and as a result, did not meet its primary endpoint.
In conclusion, Dr. Berg emphasized that some of the most important questions in advanced RCC center around first-line combination therapy, treatment sequencing in the second-line setting, and treatment optimization to reduce toxicity. These issues will be addressed with ongoing trials including COSMIC-313, PDIGREE, and PROBE. She further emphasized the importance of novel pathways including MK-6482 and in biomarkers given selection.
Presented by: Stephanie A. Berg, DO, Loyola University Medical Center - Hematology & Medical Oncology
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021


