(UroToday.com) There are several PD-1 immune-checkpoint inhibitors including nivolumab that are approved in urothelial cancer. Recently, in the front-line setting, improved activity of combined PD-L1 and CTLA4 immune-checkpoint inhibition has been reported and a phase III trial with nivolumab plus ipilimumab is ongoing. At the American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU), Dr. Marc-Oliver Grimm and colleagues presented results of their study assessing a response-based tailored approach starting treatment with nivolumab monotherapy using nivolumab plus ipilimumab as immunotherapeutic “boost”.
Between July 2017 and April 2019, 86 patients were enrolled to the TITAN-TCC study. Patients started with nivolumab 240 mg Q2W induction. After four doses and tumor assessment at week 8 (i) responders (partial or complete response) to nivolumab monotherapy continued with maintenance while (ii) patients with stable or progressive disease received two cycles of nivolumab x3 plus ipilimumab x1 followed by another two cycles of nivolumab x1 plus ipilimumab x3 if not responding. As follows is the TITAN-TCC trial design: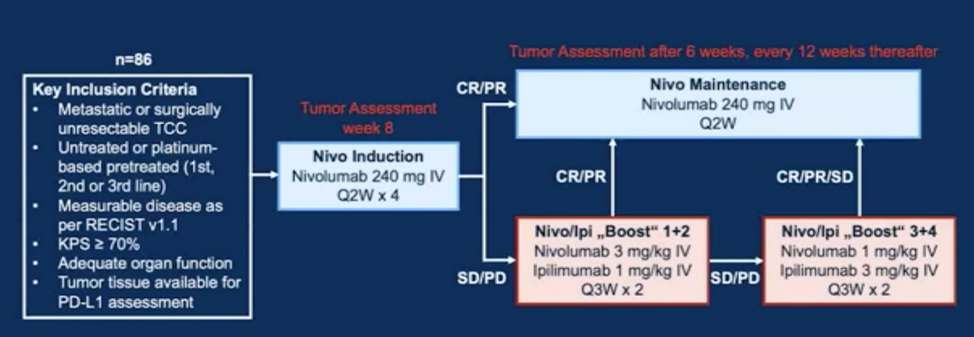
The primary endpoint was confirmed investigator-assessed objective response rate per RECIST1.1, and secondary endpoints included activity of nivolumab monotherapy at week 8, remission rate with nivolumab plus ipilimumab “boosts”, safety, overall survival and quality of life.
Among the 86 patients enrolled, 42 were first line, 39 were second line, and 5 were third line. The median age was 67 years (range 45-84), 61 patients (71 %) were male and 25 were female. Median follow-up was 7.2 months. The objective response rate with nivolumab monotherapy at first assessment (week 8) was 29% in the first line and 23% in the second/third line. There were 41 patients that received nivolumab plus ipilimumab “boosts” after week 8 while 12 received later “boosts”. The best overall response rate with nivolumab induction ± nivolumab plus ipilimumab “boosts” was 45% in the first line and 27% in the second/third line. In first line patients, 41% of patients receiving nivolumab plus ipilimumab after week 8 had an improved response compared to 8.3% in second/third line. Of the patients who continued with nivolumab maintenance after week 8 and received later “boosts,” 17% had a partial response and 17% improved to stable disease. Patients with PD-L1 >=1% treated with nivolumab +/- nivolumab plus ipilimumab in the first line had an objective response rate of 50% compared to 35% in the second/third line:
The median overall survival for first line patients was 15.1 months (95% CI 7.3-21.8) compared to 9.1 months (95% CI 5.7-18.8) for second/third line patients. The median progression-free survival for first line patients was 3.0 months (95% CI 1.8-6.8) compared to 1.9 months (95% CI 1.7-6.0) for second/third line patients.
Dr. Grimm concluded this presentation of the TITAN-TCC study with the following conclusions:
- TITAN–TCC is the first study to assess the impact of a tailored approach using ipilimumab as an immunotherapeutic boost to nivolumab monotherapy in advanced urothelial carcinoma
- Nivolumab +/- nivolumab plus ipilimumab (with escalating ipilimumab dose) shows meaningful clinical activity supporting a dual checkpoint inhibitor approach
- Patients with PD-L1 tumor cell expression >=1% had numerically a higher objective response compared to those with PD-L1 <1
- Further follow-up is ongoing to characterize duration and depth of response
Presented by: Marc-Oliver Grimm, MD, Professor and Chairman, Urology Department, Universitaetsklinikum Jena, Jena, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021


