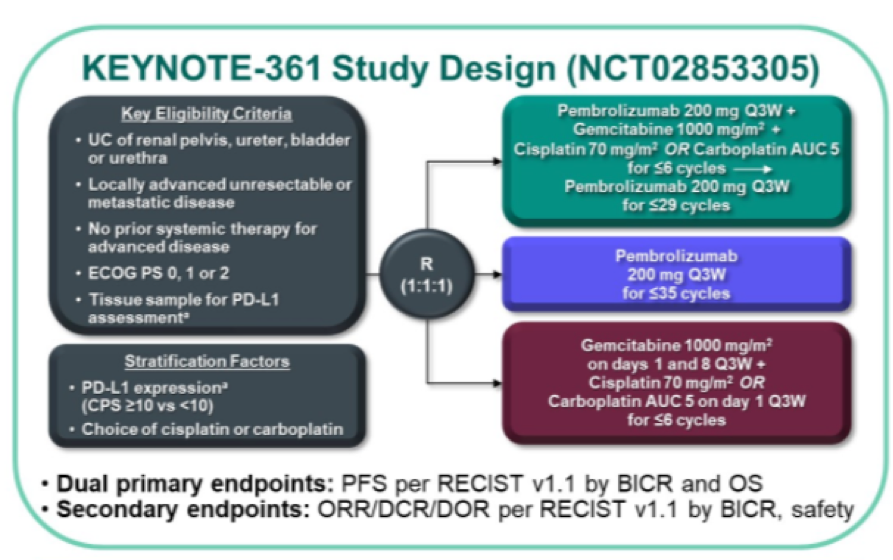
Unfortunately, as previously reported, the trial did not meet its primary endpoints, failing to demonstrate a benefit of pembrolizumab + chemotherapy vs chemotherapy alone based on progression-free or overall survival, though formal testing of overall survival for pembrolizumab vs chemotherapy was not performed. In a poster presentation at the 2021 ASCO GU Cancers Symposium, Dr. Ajjai Shivaram Alva and colleagues present an exploratory analysis of OS by subsequent therapy among patients enrolled in KEYNOTE-361 to assess how first and second-line therapy selection affected survival.
To do so, the authors examined overall survival, stratified by whether patients received subsequent therapy and by whether this therapy included an anti–PD-(L)1 agent.
Utilizing the KEYNOTE-361 cohort, the authors examined 351 patients randomized to pembrolizumab + chemotherapy, 307 patients to pembrolizumab alone, and 352 patients to chemotherapy alone with a median time from randomization to data cut-off of 32 months (22-42 months).
With a data cut-off of April 29, 2020, subsequent therapy was administered to 124 patients (35%) in the pembrolizumab + chemotherapy arm, 126 patients (41%) in the pembrolizumab alone arm, and 215 patients (61%) in the chemotherapy alone arm. Rates were similar among patients who experienced progressive disease according to blinded independent central review: 32% among those in the pembrolizumab + chemotherapy arm, 43% in the pembrolizumab alone arm, and 59% in the chemotherapy alone arm.

As may be expected, use of subsequent anti–PD-(L)1 therapy was higher among patients who initially received chemotherapy alone (48%) as compared to those in the pembrolizumab + chemotherapy arm (7%) or the pembrolizumab arm (5%). The authors then excluded patients in the pembrolizumab + chemotherapy arm and the pembrolizumab arm who received subsequent anti−PD-(L)1 therapy.
In total, the authors identified 465 patients (46%) who received second-line therapy. Among patients who did not receive anti−PD-(L)1 therapy in this setting, single-agent or combination chemotherapy was most commonly used including carboplatin, cisplatin, docetaxel, doxorubicin, gemcitabine, and paclitaxel.
Patients who initially received chemotherapy followed by second-line immunotherapy have longer overall survival (median 19 months) than those who received first-line pembrolizumab followed by a non-IO second-line approach (median 16 months).

In conclusion, the authors find that this exploratory analysis suggests that the sequence of initial chemotherapy followed by anti–PD-(L)1 agent may optimize outcomes and emphasizes the role of second-line immunotherapy.
Presented by: Ajjai Shivaram Alva, MBBS, MD Associate Professor of Medicine and a Medical Oncologist at the University of Michigan Medicine Urology Oncology Clinic, Rogel Cancer Center, Ann Arbor, MI
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter during the 2021 ASCO Genitourinary Cancers Symposium (ASCO GU), February 11th to 13th, 2021
Related Content:
The Final Results of KEYNOTE-361 - Ajjai Alva


