GALAHAD is a phase 2 study of niraparib in men with mCRPC and bi-allelic DNA damage repair (DDR) defects. Men were required to have progressed on/after at least one line of taxane-based chemotherapy and one line of androgen receptor targeting therapy. No prior treatment with a PARP inhibitor of platinum-based chemotherapy was allowed. CTCs were collected at baseline and subsequent follow-up timepoints. In this analysis, investigators analyzed CTC correlative data in the BRCA1/2 altered patients from the GALAHAD cohort.
CTC conversion was seen in 49% of overall participants. CTC0 and CTC conversion (as shown below) were early indicators of response and were associated with longer time on therapy in patients with measurable or non-measurable disease.
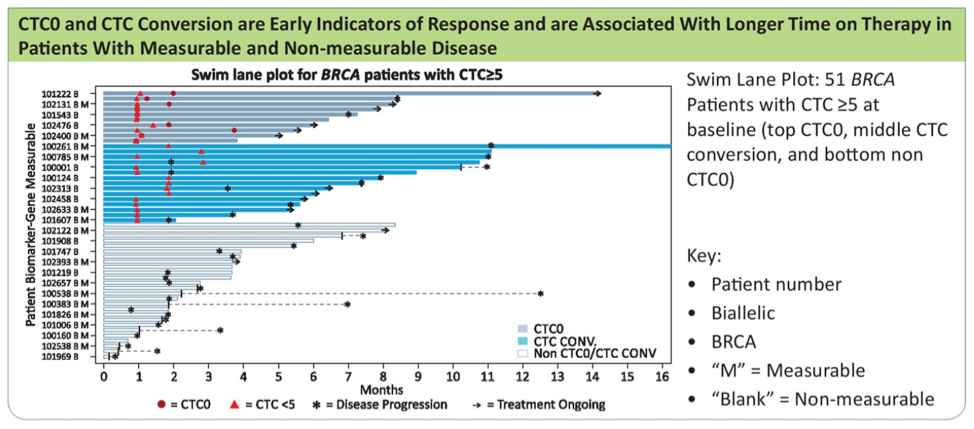
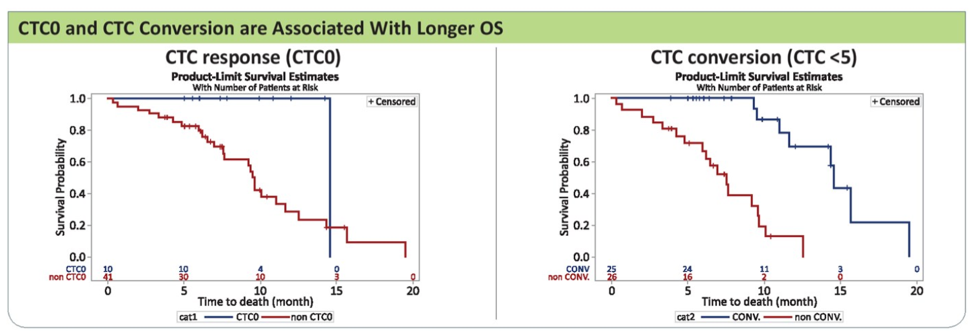
Further, CTC0 and CTC conversion were both associated with longer overall survival.
CTC0 and CTC conversion was also associated with greater reduction in PSA: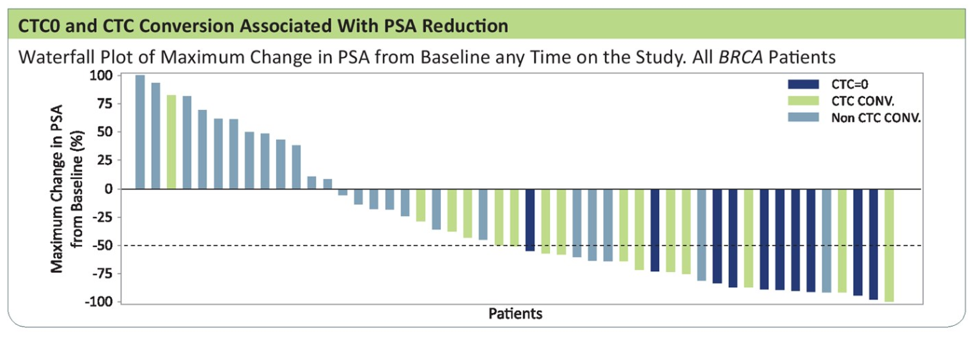
The authors concluded that:
- CTC0 and CTC conversion should be considered as a valid endpoint regardless of measurable or non-measurable disease in mCRPC
- CTC0 and CTC conversion occur early in treatment and could serve as a surrogate endpoint for long-term benefit to support accelerated drug development in patients with mCRPC
Presented by: Dr. Matthew R. Smith is Director of the Genitourinary Oncology Program at Massachusetts General Hospital Cancer Center and an Associate Professor of Medicine at Harvard Medical School
Written by: Jacob Berchuck, MD, Medical Oncology Fellow at the Dana-Farber Cancer Institute (Twitter: @jberchuck) at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California

