Interestingly, DNA repair defects were identified in 23% of metastatic castration-resistant prostate cancer patients. Additionally, 8% of metastatic castrate-resistant prostate cancer patients harbored germline mutations with 13% of them being BRCA2.1
At the ESMO 2019 meeting, Dr. de Bono presented the PROFOUND study showing alterations in homologous recombination genes in over 4000 tumor samples (Figure 1). The prevalence of germline mutations in DDR genes was shown to be 12% in metastatic prostate cancer patients, compared to 5% in patients with early prostate cancer and 3% in the general population.
Figure 1 -PROFOUND study:
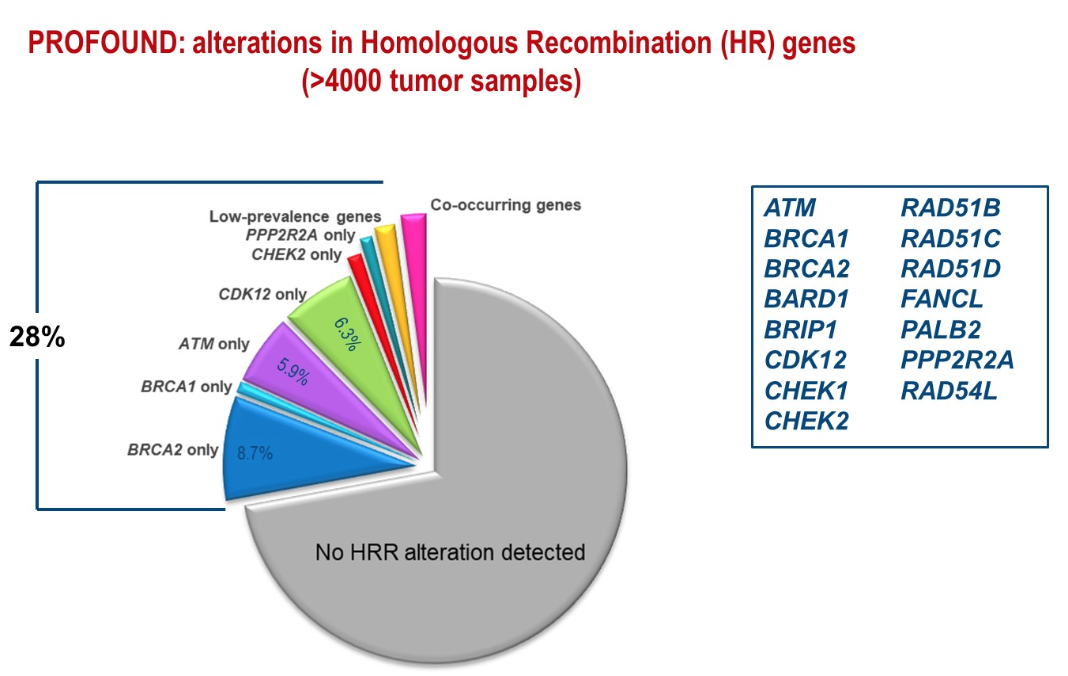
Interestingly, 30 to 40% of BRCA2 carriers lack a family history of cancer. Metastatic prostate cancer can be the whistleblower of an inherited cancer predisposition syndrome. In fact, the current NCCN guidelines recommend genetic and molecular biomarker analysis for every patient with advanced prostate cancer (Table 1).
Table 1 -NCCN guidelines recommendations regarding genetic testing and molecular biomarkers:

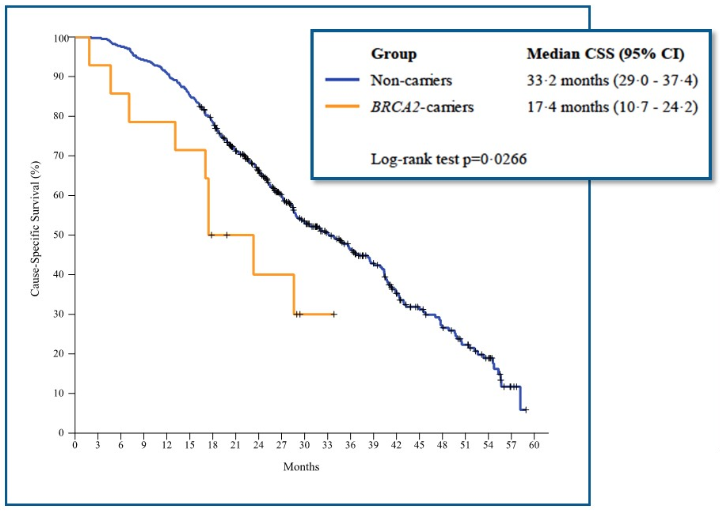
Next, Dr. Castro discussed the impact of DDR alterations in advanced prostate cancer disease. It is currently unknown what prognostic factor the somatic DDR mutations have and what their impact is on response to currently approved therapies. However, we do know that germline DDR mutations have a prognostic effect. Patients with germline DDR mutations have a shorter time from the beginning of continuous ADT until transitioning to the state of metastatic castrate-resistant prostate cancer (Figure 2). Germline DDR mutations also worsen survival in patients with metastatic castration-resistant prostate cancer (Figure 3).
Figure 2 – Effect of germline DDR mutations on the time from initiating continuous ADT until transitioning to metastatic Castrate resistant prostate cancer:

Figure 3 - The effect of germline DDR mutations on survival from mCRPC :
Both somatic and germline DDR mutations have been shown to be predictive of response to PARP inhibitors. This has been shown in several trials assessing PARP inhibitors in monotherapy of mCRPC (Table 1).
Table 1 - Studies of PARP inhibitors as monotherapy for metastatic castrate-resistant prostate cancer

In the PROFOUND phase 3 trial of Olaparib radiographic progression-free survival in patients with alterations in ATM, BRCA, BRCA2 has been shown to be better with PARP inhibitors (Figure 4). In the Triton 2 and GALAHAD trials the response to PARP inhibitors in patients with BRCA 1 and 2 alterations was shown to be significantly better (Figure 5). In contrast, the response was significantly lower in patients without BRCA1 or BRCA 2 alterations (Figure 6).
Figure 4 - PROFOUND trial showing benefit of PARP inhibitors :

Figure 5 – Response to PARP inhibitors in patients with BRCA 1 or 2 alterations:

Figure 6 - Response to PARP inhibitors in patients without BRCA 1 or 2 alterations:

Dr. Castro ended her talk stating that tumor profiling may not identify some germline mutations. This can be due to acquired changes in that tumor which can mask a point mutation in the germline. Alternatively, this can be since some tumor testing platforms filter out germline variants. Other potential reasons include the possibility that the test may not analyze genes completely and that some genes associated with cancer predisposition syndromes, may not be included in tumor genomic testing panels.
Presented by: Elena Castro, MD, PhD, Spanish National Cancer Research Centre, Madrid, Spain
Written By: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA @GoldbergHanan at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California
References:
1. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015; 161(5): 1215-28.

Both somatic and germline DDR mutations have been shown to be predictive of response to PARP inhibitors. This has been shown in several trials assessing PARP inhibitors in monotherapy of mCRPC (Table 1).
Table 1 - Studies of PARP inhibitors as monotherapy for metastatic castrate-resistant prostate cancer

In the PROFOUND phase 3 trial of Olaparib radiographic progression-free survival in patients with alterations in ATM, BRCA, BRCA2 has been shown to be better with PARP inhibitors (Figure 4). In the Triton 2 and GALAHAD trials the response to PARP inhibitors in patients with BRCA 1 and 2 alterations was shown to be significantly better (Figure 5). In contrast, the response was significantly lower in patients without BRCA1 or BRCA 2 alterations (Figure 6).
Figure 4 - PROFOUND trial showing benefit of PARP inhibitors :

Figure 5 – Response to PARP inhibitors in patients with BRCA 1 or 2 alterations:

Figure 6 - Response to PARP inhibitors in patients without BRCA 1 or 2 alterations:

Dr. Castro ended her talk stating that tumor profiling may not identify some germline mutations. This can be due to acquired changes in that tumor which can mask a point mutation in the germline. Alternatively, this can be since some tumor testing platforms filter out germline variants. Other potential reasons include the possibility that the test may not analyze genes completely and that some genes associated with cancer predisposition syndromes, may not be included in tumor genomic testing panels.
Presented by: Elena Castro, MD, PhD, Spanish National Cancer Research Centre, Madrid, Spain
Written By: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA @GoldbergHanan at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California
References:
1. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015; 161(5): 1215-28.


