San Francisco, California (UroToday.com) Recent years have seen rapid advancements in the therapy for advanced clear cell renal cell carcinoma (accRCC) with the introduction of first-line combination immunotherapy and immunotherapy/targeted therapy approached. The CheckMate 025 study, previously reported in the New England Journal of Medicine by Dr. Robert Motzer and colleagues in 2015, was a pivotal 2nd-line study of patients with accRCC who had progressed after prior anti-angiogenic therapy. This study firmly established nivolumab as a standard 2nd-line therapy in this patient population. In the Rapid Abstract Session, Dr. Motzer presented the final analysis of CheckMate 025 with over five years of follow-up.
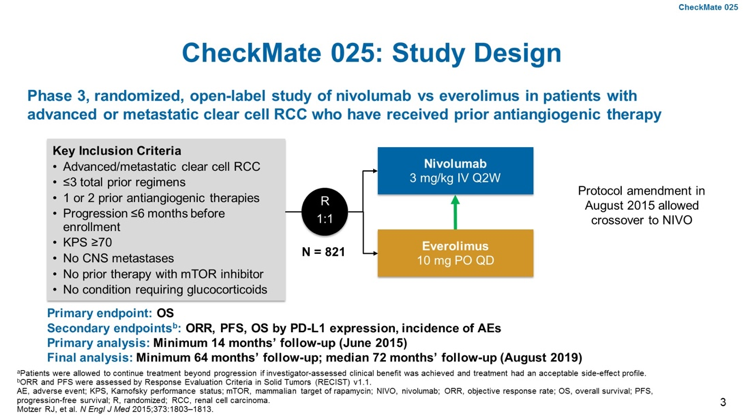
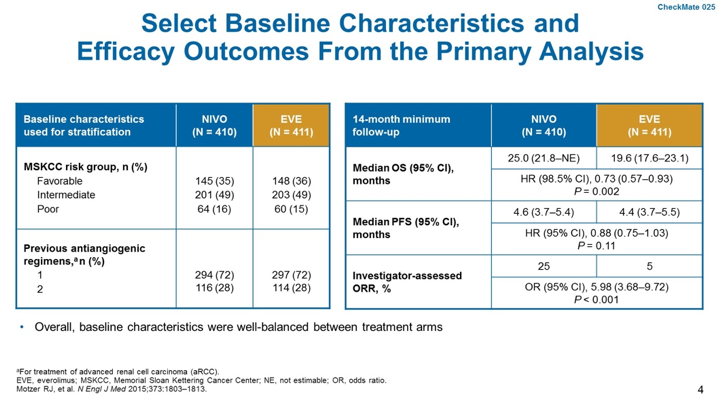
CheckMate 025 was a randomized Phase III trial of patients with locally advanced or metastatic ccRCC who had 1-2 prior lines of anti-angiogenic therapy. Patients were randomized to nivolumab 3mg/kg 2-weekly or everolimus 10mg daily. The primary endpoint of the trial was overall survival (OS) with key secondary endpoints of overall response rate (ORR), progression-free survival (PFS), safety and overall survival (OS) by PD-L1 expression. A total of 821 patients were enrolled and included representation of favorable, intermediate and poor-risk disease. Most patients (~72%) had progressed on one prior line of anti-angiogenic therapy. Generally, baseline characteristics were balanced between both arms.
With long-term follow-up, nivolumab was associated with improved OS, with a median OS of 25.8 months versus 19 months, HR 0.73, p<0.0001. Progression-free survival was also improved with nivolumab (4.2 months versus 4.5 months, HR 0.84, p=0.03).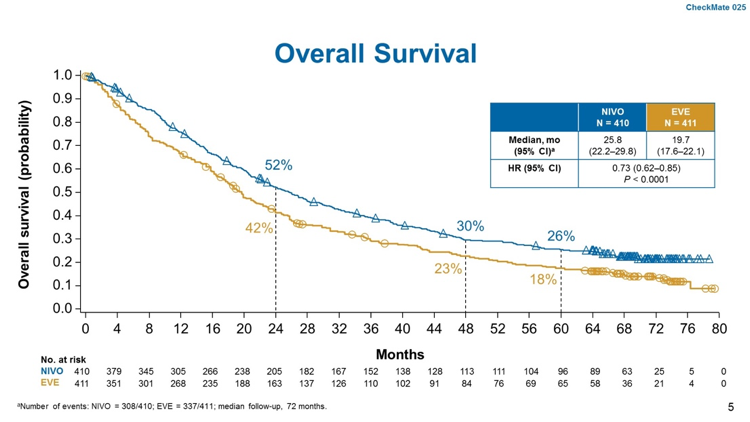
An overall response rate of 23% was observed with nivolumab, compared to 17% with everolimus (p<0.0001) and nivolumab was also associated with prolonged duration of response (18.2 months).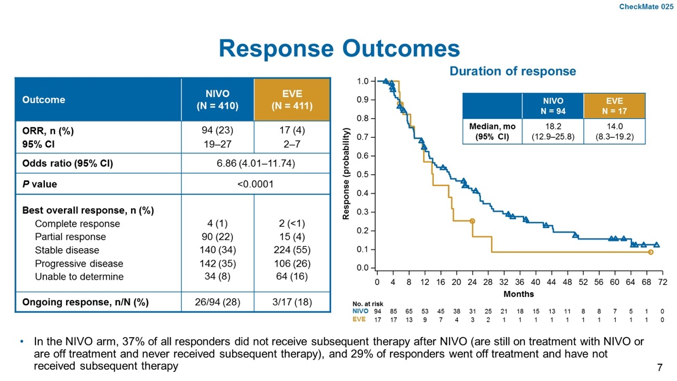
Dr. Motzer reiterated the generally safe and tolerable safety profile of nivolumab with low rates of high-grade (grade 3 or 4) toxicity. Common adverse events with nivolumab included fatigue, diarrhea, pruritis and decreased appetite. In addition, health-related quality of life analysis revealed durable improvement in scores from baseline in the nivolumab arm, compared with everolimus which showed a trend to worsening over time.
In summarizing these findings, Dr. Motzer concluded that nivolumab shows improvements in OS, PFS, response and quality of life compared with everolimus. Nivolumab monotherapy is associated with a lower proportion of treatment-related adverse events. Overall these data confirm the efficacy and safety of nivolumab in this setting.
Presented by: Robert J Motzer, MD, Medical Oncologist, Kidney Cancer Section Head, Genitourinary Oncology Service, Jack and Dorothy Byrne Chair in Clinical Oncology, Memorial Sloan Kettering Cancer Center, New York, New York
Written by: Anis Hamid, MBBS, Medical Oncology Research Fellow at Dana-Farber Cancer Institute and Medical Oncologist, PhD candidate, University of Melbourne, Melbourne, Australia, Twitter: @anis_a_hamid at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California


