San Francisco, CA (UroToday.com) Tyrosine kinase inhibitors (TKI) and the checkpoint inhibitor nivolumab (NIVO) have been shown to be significant components of systemic therapies in metastatic renal cell carcinoma. In the presented poster the authors examined whether TKI induction followed by an early switch to NIVO improved outcome in metastatic renal cell carcinoma patients.
The presented study was the NIVOSWITCH trial, which was an open-label, randomized, Phase II trial. The trial was recruiting patients between 2016 and was prematurely closed on Aug 2018 due to low accrual rate.
In this presentation, the authors showed the data of the key secondary endpoints, tumor response and derived efficacy measures.
All included patients had measurable advanced or metastatic clear cell renal cell carcinoma with an ECOG performance status between zero and two, adequate organ function, and partial response or stable disease after sunitinib or pazopanib therapy for 10 to 12 weeks.
Patients were randomized either to continue TKI treatment or receive NIVO every two to four weeks until disease progression or intolerance.
The median duration of follow up after randomization was 12.9 months. The median age was 65 years. The response to TKI induction was partial response in 59% of the patients and stable disease in 41%. In the intention to treat population, best overall response rate measured from the start of induction therapy was not significantly different in both arms (64 vs. 70%, p=0.76). However, when measured from the time of randomization, the response rate for NIVO was 16% versus 48% for TKI, p=0.029.
Table one demonstrates the patient demographics with approximately 3/4 of the patients having disseminated disease at study entry. However, there is a marked imbalance in favor of the NIVO arm, containing almost all the patients with locoregional disease only (Table 2). Antineoplastic treatment that was given before the beginning of TKI induction is elaborated in Table 3. As shown, most patients had undergone surgery. Table 4 elaborates on the induction therapy that patients had received in both study arms.
Table 1. Patient demographics
Table 2. Current staging: M category
Table 3. Pre-treatment for renal cancer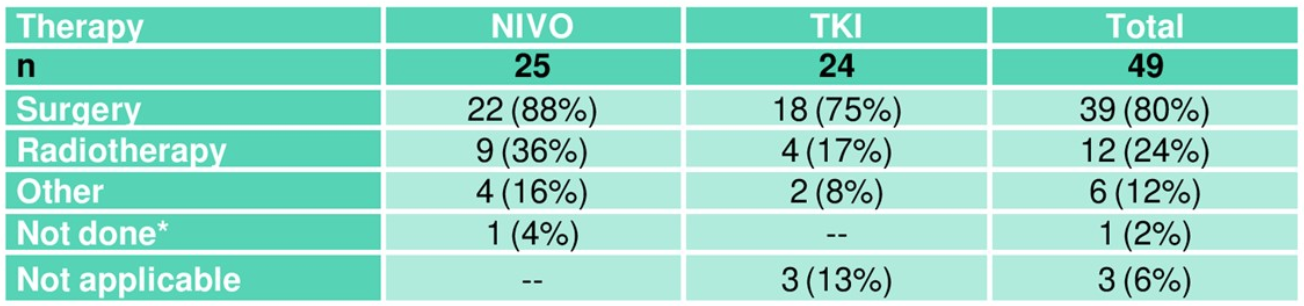
Table 4. Induction therapy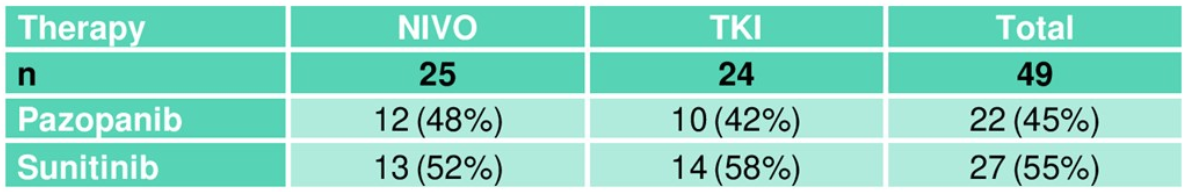
The results from re-staging after randomization are shown in Table 5, indicating a much higher rate of early progression after randomization in arm A (NIVO). The objective response rate after randomization is 16% in the NIVO arm compared to 48% in the standard TKI arm. The Kaplan Meier curve of progression-free survival showing a highly significant difference in favor of standard arm B (TKI) is shown in Figure 1.
Table 5. Overall best response after randomization (ITT)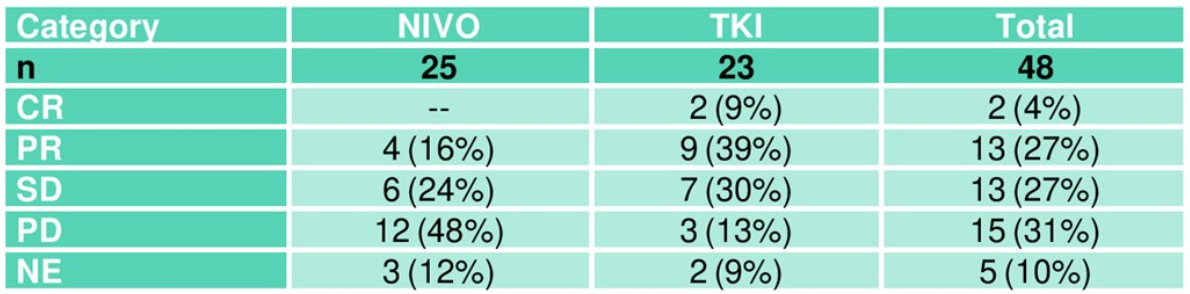
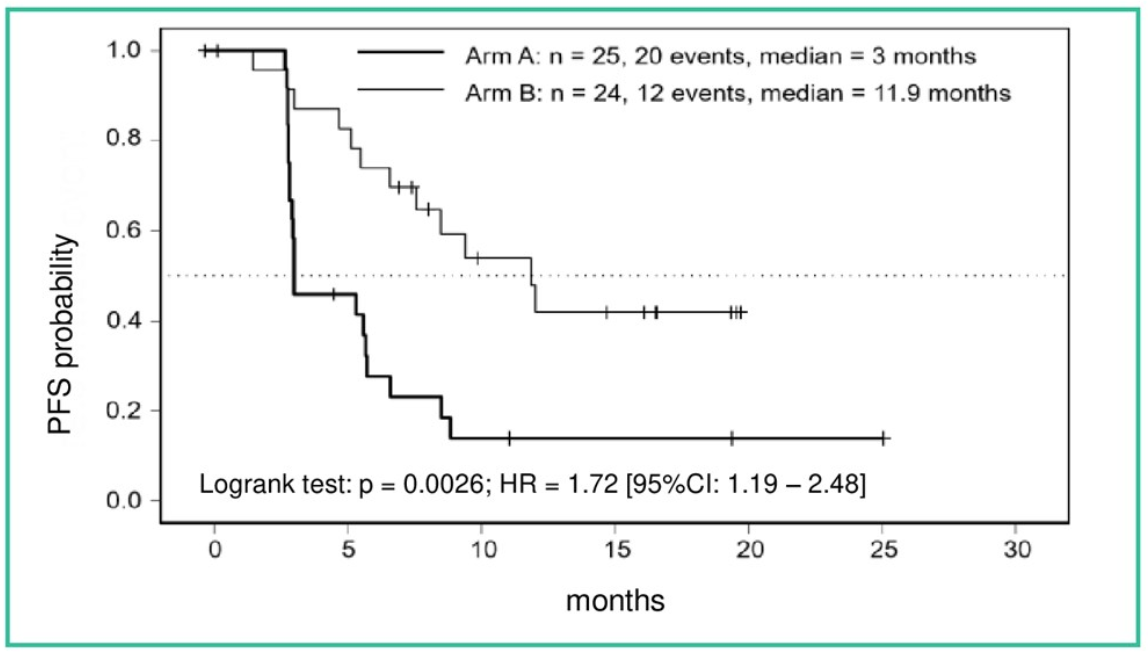
Figure 1. PFS from randomization
The median follow-up was of 12.9 months, but the median overall survival was not reached as only 9 events had occurred by the time the trial ended. However, a trend in favor of TKI continuation was shown (Figure 2). Table 6 elaborates on the adverse events in both trial arms.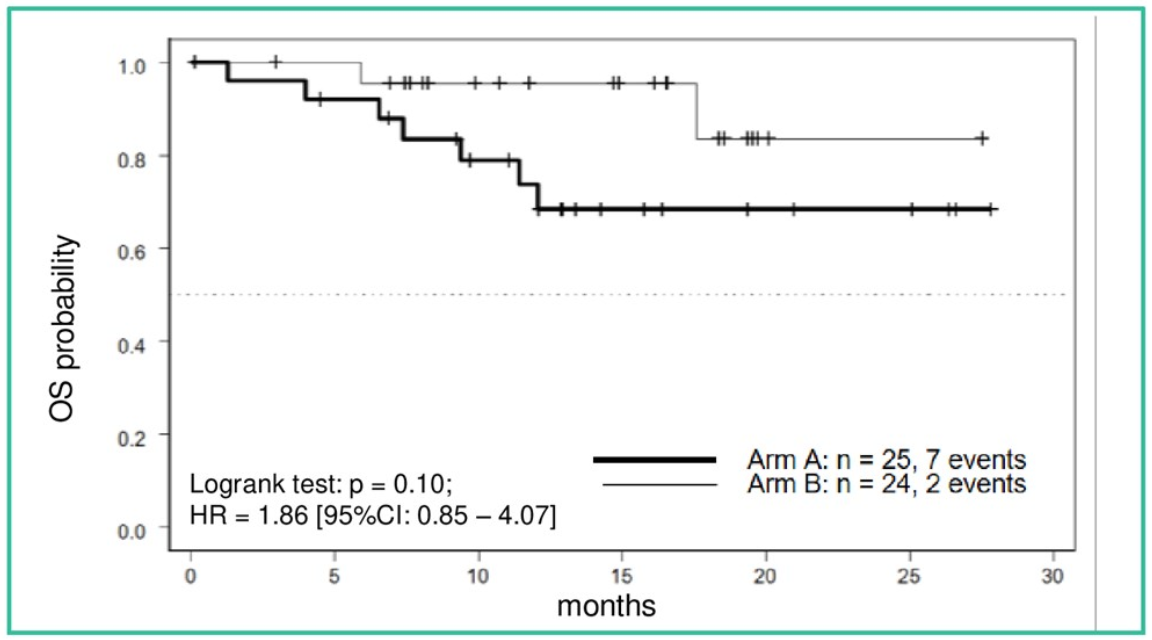
Figure 2. OS from randomization
Table 6. Adverse events (overview)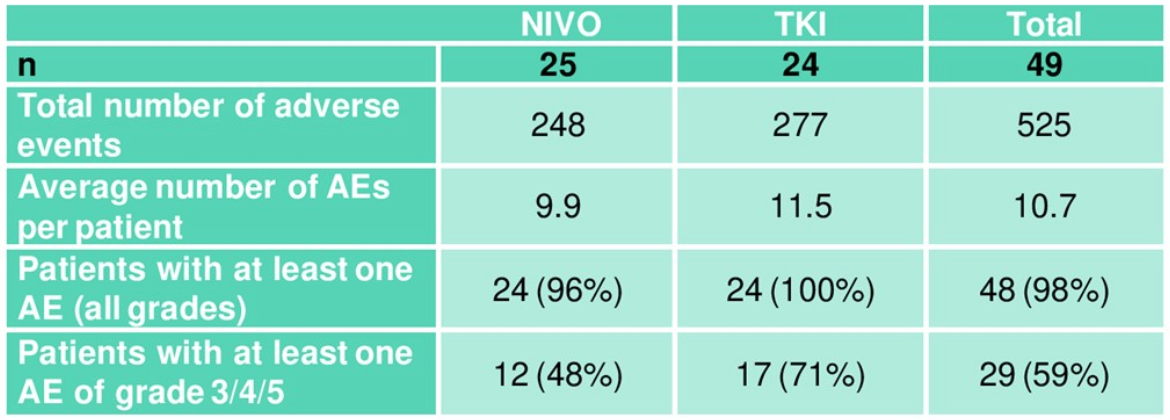
The authors concluded that TKI induction followed by an early switch to NIVO did not improve the objective response rate in patients responsive to TKI initially. Therefore, there is no evidence to support the notion that TKI pre-treatment sensitizes for NIVO efficacy.
Presented by: Viktor Grünwald, MD, University Hospital Essen, Clinic for Hematology, Hemostasis, Oncology and Stem Cell Transplantation, Universitätsklinikum Essen, Essen, Germany
Written by: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, New York, Twitter: @GoldbergHanan at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California


